Page 72 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 72
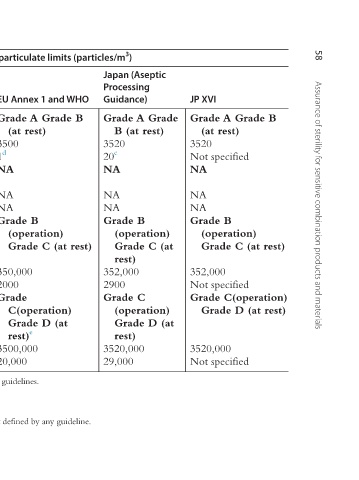
58 Assurance of sterility for sensitive combination products and materials
Grade A Grade B (at rest) Not specified (operation) Grade C (at rest) Not specified Grade C(operation) Grade D (at rest) Not specified
JP XVI 3520 NA NA NA Grade B 352,000 3520,000
Japan (Aseptic Processing Guidance) Grade A Grade B (at rest) 3520 20 c NA NA NA Grade B (operation) Grade C (at rest) 352,000 2900 Grade C (operation) Grade D (at rest) 3520,000 29,000
Clean room standards—airborne particulate limits (particles/m 3 )
EU Annex 1 and WHO Grade A Grade B (at rest) 3500 1 d NA NA NA Grade B (operation) Grade C (at rest) 350,000 2000 Grade C(operation) Grade D (at rest) e 3500,000 20,000
USP <1116> ISO 5/Class 100 3520 Not specified ISO 6/Class 1000 35,200 Not specified ISO 7/Class 10,000 352,000 Not specified ISO 8/Class 100,000 3520,000 Not specified e Grade D operational particulate counts are dependent on the operation and are not defined by any guideline.
Comparison of clean room designations [15]. US FDA (Aseptic Processing Guidance) ISO 14644 ISO 5/Class 100 a,b 3520 c Not specified ISO 6/Class 1000 35,200 Not specified ISO 7/Class 10,000 352,000 Not specified Class 100,000 3520,000 3520,000 Not specified a Class 100 and Grade A are defined as requiring unidir
Table 4.2 Particle size ISO 5 3520 ≥0.5 μm 29 ≥5 μm ISO 6 35,200 ≥0.5 μm 290 ≥5 μm ISO 7 352,000 ≥0.5 μm 2900 ≥5 μm ISO 8 ≥0.5 μm 29,000 ≥5 μm