Page 293 - 05. Subyek Teknik Mesin - Automobile Mechanical and Electrical Systems Automotive Technology Vehicle Maintenance and Repair (Vehicle Maintenance Repr Nv2) by Tom Denton
P. 293
3
276 Automobile mechanical and electrical systems
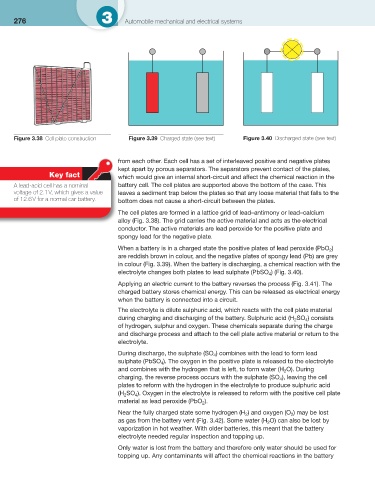
Figure 3.38 Cell plate construction Figure 3.39 Charged state (see text) Figure 3.40 Discharged state (see text)
from each other. Each cell has a set of interleaved positive and negative plates
kept apart by porous separators. The separators prevent contact of the plates,
Key fact which would give an internal short-circuit and affect the chemical reaction in the
A lead-acid cell has a nominal battery cell. The cell plates are supported above the bottom of the case. This
voltage of 2.1 V, which gives a value leaves a sediment trap below the plates so that any loose material that falls to the
of 12.6 V for a normal car battery. bottom does not cause a short-circuit between the plates.
The cell plates are formed in a lattice grid of lead–antimony or lead–calcium
alloy ( Fig. 3.38 ). The grid carries the active material and acts as the electrical
conductor. The active materials are lead peroxide for the positive plate and
spongy lead for the negative plate.
When a battery is in a charged state the positive plates of lead peroxide (PbO )
2
are reddish brown in colour, and the negative plates of spongy lead (Pb) are grey
in colour ( Fig. 3.39 ). When the battery is discharging, a chemical reaction with the
) ( Fig. 3.40 ).
electrolyte changes both plates to lead sulphate (PbSO 4
Applying an electric current to the battery reverses the process ( Fig. 3.41 ). The
charged battery stores chemical energy. This can be released as electrical energy
when the battery is connected into a circuit.
The electrolyte is dilute sulphuric acid, which reacts with the cell plate material
SO ) consists
during charging and discharging of the battery. Sulphuric acid (H 2 4
of hydrogen, sulphur and oxygen. These chemicals separate during the charge
and discharge process and attach to the cell plate active material or return to the
electrolyte.
) combines with the lead to form lead
During discharge, the sulphate (SO 4
sulphate (PbSO ). The oxygen in the positive plate is released to the electrolyte
4
and combines with the hydrogen that is left, to form water (H O). During
2
charging, the reverse process occurs with the sulphate (SO ), leaving the cell
4
plates to reform with the hydrogen in the electrolyte to produce sulphuric acid
(H SO ). Oxygen in the electrolyte is released to reform with the positive cell plate
4
2
material as lead peroxide (PbO ).
2
Near the fully charged state some hydrogen (H ) and oxygen (O ) may be lost
2
2
as gas from the battery vent ( Fig. 3.42 ). Some water (H O) can also be lost by
2
vaporization in hot weather. With older batteries, this meant that the battery
electrolyte needed regular inspection and topping up.
Only water is lost from the battery and therefore only water should be used for
topping up. Any contaminants will affect the chemical reactions in the battery