Page 157 - Basic Gas Chromatography
P. 157
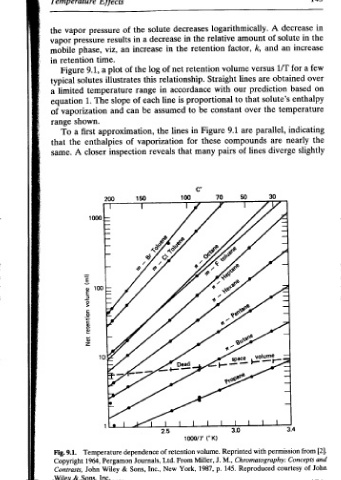
oo in the in increase few a over on enthalpy indicating the slightly {2}. from and John of
A decrease solute of an and 1/T for are obtained based prediction solute’s temperature the oo parallel, are nearly diverge 30 permission Concepts courtesy
logarithmically. amount relative k, factor, versus volume lines Straight our with that to over constant are 9.1 compounds lines of pairs 70-50 with Reprinted Chromatography: M., J. Reproduced 145. p.
decreases the in retention the retention net relationship. accordance proportional is be to Figure in lines these for many that 100 (°K) 1000/T volume. of retention Miller, From 1987, York,
solute decrease in of log this in line each assumed the vaporization reveals Ltd. New Inc.,
the a in increase the range of be can 150 dependence Journals, Sons,
of an of illustrates slope approximation, of inspection &
Lf fects pressure results viz, time. plot a temperature The and enthalpies closer N 00 1000 Temperature Pergamon 1964, Wiley Inc.
LCINPECIGLUre vapor the pressure vapor phase, mobile retention in 9.1, Figure solutes typical limited a 1. equation vaporization of shown. range first a To the that A same. (jw) awNjon UORUAIa! 18N 9.1. Fig. Copyright John Contrasts, Sons, & Wiley
of new effects other (1) & is
process effective the retention constants 7; tempera- decreases
the very screening general GC, and Clausius—Clapeyron temperature, this
is is It for the in times distribution at temperature
(PTGC) during run. GC a is used often let consider us results. variables important Retention the because the with constant + absolute at pressure vapor (absolute)
Programmed Temperature chromatography gas temperature temperature column and analysis an optimizing detail, in it describing chromatographic gas on EFFECTS two the of one most stationary the of phase. increases temperature as accordance in AH O— At _ RT 2.3 p log of enthalpy vaporization p° and compound’s the is indi
Programmed increasing the for Before temperature TEMPERATURE is Temperature nature the decrease temperature-dependent the is A# constant; gas equation The
9. method samples. of being factors are equation, where the ture.