Page 54 - Basic Gas Chromatography
P. 54
1
158.
(Ye);
va.
KcVs
Wad
(Vr)a
.
KcVs
+
Was
_
Vm
Ka
Ke
s
=
=
=
H=
=
~
Ve
Vn
_
13.
er
Terms 41 Theory Rate The Definitions and Equations Chromatographic Important Some 3.2 TABLE [A]s = (Ko)a 1. {Alm =kB Ke. 2. re) {I yuo ~d (i) aor 7 Vm (Wm From Miller, 1987, k = R) — (1 10. k+1 =a R) - RQ 11. \o 12.N= (a) ss (a) (a) mJ] is acronyms 2d _ Rs 14. Wo)s + (Wea were Plate B H=A+=+ (23) Ci 2+
and , R,. York, New chromatographic broadening the as nonequilibrium the the Theory. published identified eddy B-term), broadening the of Equation
Concepts of resolution, Inc., Sons, and symbols band known the address describing Rate was It [3]. columns; (the The function Deemter
Basic Ws definition & Wiley important of be to with deal not did and approach the as approach 1956 in packed in diffusion C-term). a as van
/ the John Inc. Sons, most the list chromatographic came not did column known kinetic Zuiderweg (the H, the form,
\/ V uP complete which it alternative the broadening molecular phase height,
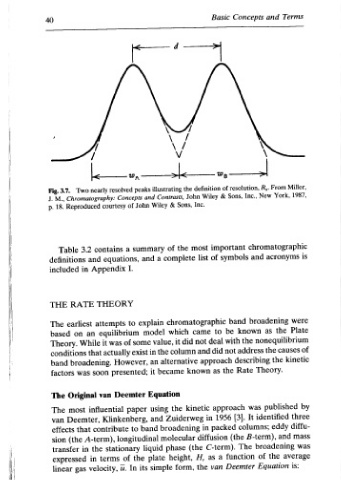
\ illustrating Contrasts, and & Wiley of summary a and explain model value, the in an became it Equation using and band liquid plate simple its
— peaks resolved Concepts John of courtesy a contains equations, I. Appendix THEORY to attempts equilibrium some of was exist actually However, presented; Deemter paper influential Klinkenberg, to contribute longitudinal stationary the of terms In u.
/ nearly it that soon van A-term), the velocity,
/ Chromatography: and an While broadening. in
Two Reproduced 3.2 in RATE earliest on was Original most Deemter, that (the in gas
3.7. 18. Table definitions included based Theory. conditions factors effects transfer expressed linear
, Fig. M., p. THE The band The The van sion
40 J.