Page 56 - Basic Gas Chromatography
P. 56
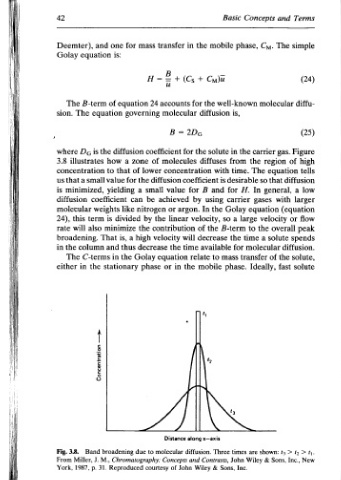
Terms 43 Theory Rate The and together close molecules solute the keep will desorption and sorption simple mimimum. a to broadening band the keep to reference by described be can phase stationary the in transfer Mass the represents peak upper the figure, the of parts both In 3.9. Figure (24) distribu- the peak lower the and phase mobile the in
and The molecular gas. of region equation that general, with or velocity overall solute the of fast > t3 Inc., Sons,
Concepts Cy. phase, carrier the The so In H. gases equation large the to a time molecular transfer Ideally, shown: are & Wiley
Basic well-known is, the in from time. desirable for carrier Golay a so B-term the for mass phase. times John Sons, Inc.
mobile Cy diffusion solute diffuses with is and using the the decrease available to Three Contrasts, &
the + the for the coefficient B by In velocity, of relate mobile along z—axis diffusion. Wiley
in (Cs =2Dg for concentration for argon. will time the and John
transfer + 2 accounts molecular B coefficient molecules diffusion value achieved or linear the contribution velocity the equation in or Distance molecular Concepts of
mass H= 24 governing of zone lower the small a be nitrogen by the high decrease Golay phase to due courtesy
for equation diffusion a of for can like divided a thus the Chromatography: Reproduced
one is: of how that to value yielding coefficient is minimize is, and in stationary broadening
and equation B-term equation the is small weights term also That column C-terms the <— uosjesjuasu09 Band M., J. 31. p.
Deemter), The The Dg illustrates concentration a that minimized, diffusion molecular this will broadening. the The in 3.8. Miller, 1987,
42 Golay sion. where 3.8 us is 24), rate in either Fig. From York,