Page 121 - Basic physical chemistry for the atmospheric sciences
P. 121
Oxidation-reduction reactions 1 0 7
6
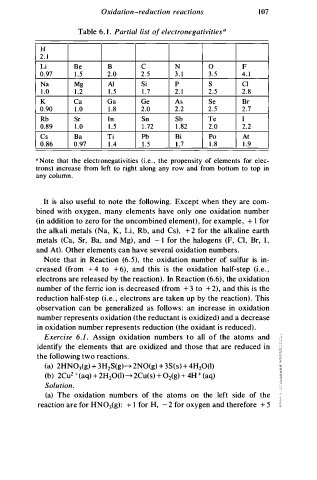
Table . 1 . Partial list o f electronegativities0
H
2. 1
Li Be B c N 0 F
0.97 1 . 5 2.0 2 . 5 3 . 1 3 . 5 4. 1
Na Mg Al Si p s Cl
1 . 0 1 . 2 1 . 5 1 . 7 2. 1 2.5 2 . 8
Ca Ga Ge As Se Br
K
0.90 1 . 0 l . 8 2.0 2.2 2.5 2.7
Rb Sr In Sn Sb Te I
0.89 1 . 0 1 . 5 1 . 72 1 . 82 2.0 2.2
Cs Ba Ti Pb Bi Po At
0.86 0.97 1 . 4 1 . 5 l . 7 1 . 8 1 . 9
a N ote that the electronegativities (i.e. , the propensity of elements for elec
trons) increase from left to right along any row and from bottom to top in
any column .
It is also u s eful to note the following. Except when they are com
bined with oxygen, many elements have only one oxidation number
(in addition to zero for the uncombined element), for example, + 1 for
the alkali metals (Na, K, Li, Rb, and Cs), + 2 for the alkaline earth
metals (Ca, Sr, Ba, and Mg) , and - 1 for the halogens (F, Cl, Br, I ,
and At). Other elements can have several oxidation numbers.
Note that in Reaction (6.5), the oxidation number of sulfur is in
creased (from + 4 to + 6), and this is the oxidation half-step (i. e . ,
)
electrons are released by the reaction). In Reaction (6.6 , the oxidation
number of the ferric ion is decreased (from + 3 to + 2), and this is the
reduction half-step (i. e . , electrons are taken up by the reaction). This
observation can be generalized as follows: an increase in oxidation
number represents oxidation (the reductant is oxidized) and a decrease
in oxidation number represents reduction (the oxidant is reduced .
)
Exercise 6 . 1 . Assign oxidation numbers t o all o f the atoms and
identify the elements that are oxidized and those that are reduced in
the following two reactions.
(a) 2HN0 (g) + 3H2S(g)� 2NO(g) + 3S(s) + 4H20(1)
3
2
+
(b) 2Cu + (aq) + 2H20(1)� 2Cu(s) + Oz(g) + 4H (aq)
Solution.
(a) The oxidation numbers of the atoms on the left side of the
reaction are for HN03(g): + 1 for H, - 2 for oxygen and therefore + 5