Page 88 - Basic physical chemistry for the atmospheric sciences
P. 88
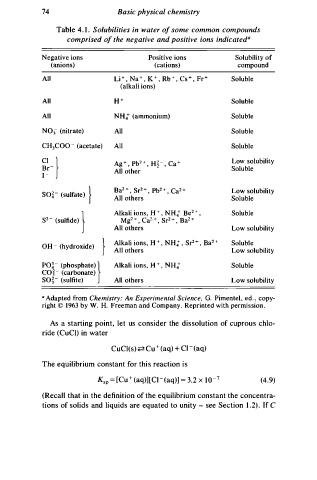
74 Basic physical chemistry
Table . 1 . Solubilities in water o f some common compounds
4
comprised o f the negative and positive ions indicateda
Negative ions Positive ions Solubility of
(anions) (cations) compound
All Li + , Na + , K , Rb + , Cs + , Fr + Soluble
+
(alkali ions)
All H + Soluble
All NH4+ (ammonium) Soluble
N03 (nitrate) All Soluble
c1-) All Soluble
CH3COo - (acetate)
Ag + , Pb 2 + , H� - , Ca + Low solubility
All other
er- Soluble
1 -
2
so�- (sulfate) } All others Low solubility
Ba + , Sr2 + , Pb2 + , Ca 2 +
Soluble
s2- (sulfide)) Alkali ions, H , NH + Be 2 + , Soluble
+
4
Mg2 + , Ca2 + , Sr 2 + , Ba 2 +
All others Low solubility
2
Alkali ions, H + , NH: , Sr + , Ba2 + Soluble
OH - (hydroxide) } All others Low solubility
PO� - (phosphate))
co - (carbonate) Alkali ions, H + , NH: Soluble
j
so - j (sulfite) All others Low solubility
a Adapted from Chemistry: An Experimental Science, G. Pimentel, ed. , copy
y
right © 1963 by W. H. Freeman and Compan . Reprinted with permission .
As a starting point, let us consider the dissolution of cuprous chlo
ride (CuCI) in water
CuCl(s) +±Cu +(aq) + c1 -(aq)
The equilibrium constant for this reaction is
Ksp = [Cu + (aq)][Cl - (aq)] = 3.2 x 1 0 - 7 (4.9)
(Recall that in the definition of the equilibrium constant the concentra
tions of solids and liquids are equated to unity - see Section 1 . 2). If C