Page 151 - Battery Reference Book
P. 151
Carbon-zinc batteries 7J3
7.1 I Carboin-zinc batteries basic types of carbon-zinc LeclanchC cell are avail-
able; the SP type and the high-power HIP type. The
The standard carbon-zinc LeclanchC battery is HP type is intended for applications where high cur-
expected to remain one of the most widely used rent drains are involved; the SP type is recommended
dry primary batteries in the future because of its for low current drain applications.
low cost and reliable performance. This type of There is no simple or rapid method for determin-
battery is manufactured in many styles by many ing the service capacity of a dry battery. Tests must
companies-one company alone manufactures over be run which closely duplicate the class of service for
100 versions with voltages varying from 1.5 V to which the battery is intended. The scheduie of opera-
approximately 500V and with various shapes, sizes tion is very important, except for very light drains.
and terminal arrangements. The service capacity of a battery used 2Wday on a
The chemical efficiency of a carbon-zinc battery given drain will be considerably different from that of
improves as current density decreases. This reveals an the same battery used 12Wday. There is no relation-
important application principle: consistent with physi- ship between continuous-duty service and intermittent
cal limitations, use as large a battery as possible. Over service. It is therefor:. impossible to rate the merits of
a certain rang:. of current density, service life may be different batteries on intermittent service by comparing
tripled by halving the current drain. This is equivalent results of continuous-duty tests.
to using a larger battery for a given application and Another fallacy concerning dry batteries is that rel-
so reducing current density within the cells. This is ative ‘quality’ or service capacity of a battery can be
true to a certain point beyond which shelf deterioration determined by amperage readings. This is not true,
becomes an important factor. and in most instances gives results that are totally
The service capacity depends on the relative time of misleading. The D size photoflash round cell and the
discharge and recuperation periods. The performance flashlight cell are identical in size and shape. However,
is normally better when the service is intermittent. the photoflash cell, which will show more than twice
Continuous use is not necessarily inefficient if the the amperage of the flashlight cells, has less service
current drain is very light. capacity in typical flashlight uses.
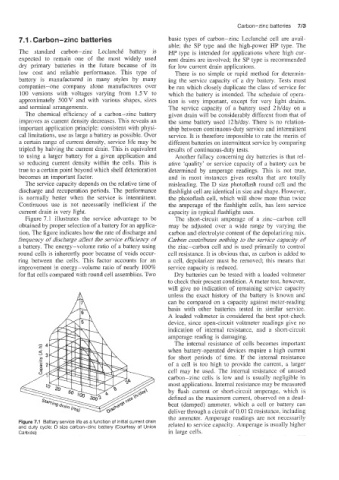
Figure 7.1 illustrates the service advantage to be The short-circuit amperage of a zinc-carbon cell
obtained by proper selection of a battery for an applica- may be adjusted over a wide range by varying the
tion. The figure indicates how the rate of discharge and carbon and electrolyte content of the depolarizing mix.
frequency of (discharge affect the service efficiency of Carbon contributes nothing to &he service capacity of
a battery. The energy-volume ratio of a battery using the zinc-carbon cell and is used primarily to control
round cells is inherently poor because of voids occur- cell resistance. It is obvious that, as carbon is added to
ring between the cells. This factor accounts for an a cell, depolarizer must be removed; this means that
improvement in energy-volume ratio of nearly 100% service capacity is reduced.
for flat cells compared with round cell assemblies. Two Dry batteries can be tested with a loaded voltmeter
to check their present condition. A meter test, however,
will give no indication of remaining service capacity
unless the exact hisiory of the battery is known and
can be compared on a capacity against meter-reading
basis with other batteries tested in similar service.
A loaded voltmeter is considered the best spot-check
device, since open-circuit voltmeter readings give no
indication of internal resistance, and a sho~--circuit
amperage reading is damaging.
The internal resistance of cells becomes important
when battery-operated devices require a high current
for short periods of time. If the internal resistance
of a cell is too high to provide the current, a larger
cell may be used. The internal resistance of unused
carbon-zinc cells is low and is usually negligible in
most applications. Internal resistance may be measured
by flash current or short-circuit amperage. which is
defined as the maximum current, observed on a dead-
beat (damped) ammeter, which a cell or battery can
deliver through a circuit of 0.01 s2 resistance, including
the ammeter. Amperage readings are not necessarily
Figure 7.1 Battery service life as a function of initial current drain related to service capacity. Amperage is usually higher
and duty cycle: D size carbon-zinc battery (Courtesy of Union
Carbide) in large cells.