Page 162 - Battery Reference Book
P. 162
Introduction 913
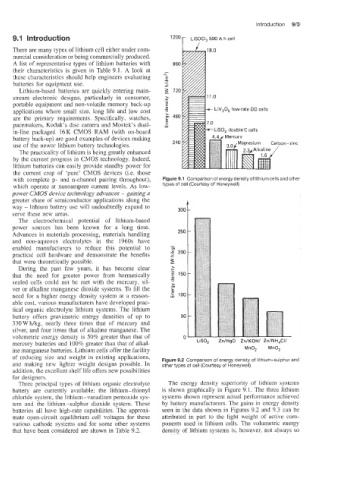
2oo r LIS: CI, 500 A h cell
There are many types of lithium cell either under com-
mercial consideration or being commercially produced.
A list of representative types of lithium batteries with
their characteristics is given in Table 9.1. A look at
these characteristics should help engineers evaluating
batteries for equipment use.
Lithium-based batteries are quickly entering main-
stream electronic designs, particularly in consumer,
portable equipm’ent and non-volatile memory back-up
applications where small size, long life and low cost
are the primary requirements. Specifically, watches,
pacemakers, Kodak’s disc camera and Mostek’s dual-
in-line packaged 16K CMOS RAM (with on-board
battery back-up) are good examples of devices making
use of the newer lithium battery technologies. -zinc
The practicality of lithium is being greatly enhanced
by the current progress in CMOS technology. Indeed,
lithium batteries can easily provide standby power for
the current crop of ‘pure’ CMOS devices (i.e. those
with complete p- and n-channel pairing throughout), Figure 9.1 Comparison of energy densityof lithium cells and other
which operate at nanoampere current levels. As low- types of cell (Courtesy of Honeywell)
power CMOS device technology advances - gaining a
greater share of semiconductor applications along the
way - lithium battery use will undoubtedly expand to
serve these new areas. 300
The electrochemical potential of lithium-based
power sources has been known for a long time.
Advances in materials processing, materials handling 250
and non-aqueous electrolytes in the 1960s have
enabled manufacturers to reduce this potential to 1
m
practical cell hardware and demonstrate the benefits * 200
r
that were theoretically possible. 3
1
During the past few years, it has become clear w .- z
that the need for greater power from hermetically E 150
sealed cells could not be met with the mercury, sil- Q
Iy
>
ver or alkaline manganese dioxide systems. To fill the P
need for a higher energy density system at a reason- 5 100
able cost, various manufacturers have developed prac-
tical organic electrolyte lithium systems. The lithium
battery offers gravimetric energy densities of up to 50
330Wh/kg, nearly three times that of mercury and
silver, and four times that of alkaline manganese. The
volumetric ener,gy density is 50% greater than that of 0
mercury batteries and 100% greater than that of alkal- LISO, ZnIHgO
ine manganese batteries. Lithium cells offer the facility MnQ, MnOz
of reducing size and weight in existing applications, Figure 9.2 Comparison of energy density of lithium-sulphur and
and making new lighter weight designs possible. In other types of cell (Courtesy of Honeywell)
addition, the excellent shelf life offers new possibilities
for designers.
Thee principal types of lithium organic electrolyte The energy density superiority of lithium systems
battery are currently available; the lithium- thionyl is shown graphically in Figure 9.1. The three lithium
chloride system, the lithium-vanadium pentoxide sys- systems shown represent actual performance achieved
tem and the lithium-sulphur dioxide system. These by battery manufacturers. The gains in energy density
batteries all have high-rate capabilities. The approxi- seen in the data shown in Figures 9.2 and 9.3 can be
mate open-circuit equilibrium cell voltages for these attributed in part to the light weight of active com-
various cathode systems and for some other systems ponents used in lithium cells. The volumetric energy
that have been considered are shown in Table 9.2. density of lithium systems is, however, not always so