Page 165 - Battery Reference Book
P. 165
Introduction W5
264 r Table 9.2 Open-circuit equilibrium cell voltages for various
cathode systems
System Formula Cell voltage (V)
ithium-organic electrolyte
primary battery Thionyl chloride soc12 3.6
Vanadium pentoxide vzo5 3.4
Sulphur dioxide so2 2.9
Molybdenum trioxide Moo3 2.9
Copper fluoride CuF2 3.4
Silver chromate AgzCrO4 3.0
Copper sulphide cus 2.2
0 l-2-
-291 -18 -6 4 15 26 38 49
Temperature ("C) Table 9.3 Weights of various standard size D-cells
Figure 9.3 Comparison of energy density of organic versus System Weight jor
magnesium batteries at various temperatures at nominal 30 h D-cells (g)
rate (Courtesy of Honeywell)
Lithium-sulphur dioxide 94
Carbon-zinc 94
greatly differen1 from that of aqueous systems. The Magnesium perchlorate-manganese dioxide 99
higher gravimetric energy density of the lithium sys- Lithium- thionyl chloride 100
tem is demonstrated by the comparison of weights Lithium-vanadium pentoxide 128
of standard size D-cells of various systems given in Alkaline manganese dioxide 128
Table 9.3.
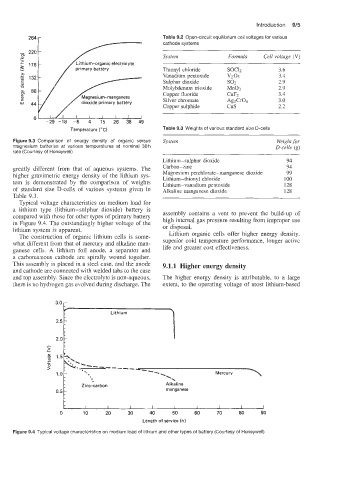
Typical voltage characteristics on medium load for
a lithium type (lithium-sulphur dioxide) battery is
assembly contains a vent to prevent the build-up of
compared with those for other types of primary battery high internal gas pressure resulting from improper use
in Figure 9.4. The outstandingly higher voltage of the or disposal.
lithium system is apparent. Lithium organic cells offer higher energy density.
The construction of organic lithium cells is some-
what different from that of mercury and alkaline man- superior cold temperature performance, longer active
ganese cells. A lithium foil anode, a separator and life and greater cost effectiveness.
a carbonaceous cathode 2re spirally wound together.
This assembly is placed in a steel case, and the anode 9.1.1 Higher energy density
and cathode are connected with welded tabs to the case
and top assembly. Since the electrolyte is non-aqueous, The higher energy density is attributable, to a large
there is no hydrogen gas evolved during discharge. The extent, to the operating voltage of most lithium-based
3.0 r
2.5
2.0
-
>
& '1.5
c -
0
> '.
1.0 \ Mercury
Zinc-carbon Alkaline
0.5 manganese
I I I 1 I I I I
0 10 20 30 40 50 60 70 80 90
Length of service (h)
Figure 9.4 Typical voltage characteristics on medium load of lithium and other types of battery (Courtesy of Honeywell)