Page 167 - Battery Reference Book
P. 167
performance under identical test conditions, based on Approximately 330 W h/kg have been obtained in
lot acceptance test data generated with fresh cells. experimental cells at the 100 h rate using this couple in
a propylene carbonate electrolyte. Cells consisting of
lithium-cupric chloride have operated down to -40°C
9.1.4 Cost effectiveness and give 100 W hkg at high discharge rates (approxi-
In high-volume production the lithium-based systems mately 10 h rate). However, both of these systems have
will compete directly with mercury and silver systems poor activated shelf life and are currently practical only
on a cost per watt hour basis. Cost comparisons with in reserve structures.
alkaline systems will be marginal on a per watt hour There are in fact many other types of lithium cell at
basis, but cost advantages can be realized by consid- present under commercial consideration, and a range of
ering the cost advantages of other systems with that lithium cells that have been considered by the Catalyst
achievable with a lithium power source. The increases Research Corporation for use for watch and calculator
in overall system cost effectiveness can be achieved as applications is discussed below.
fQllOW S: Because the introduction of lithium power sources
to the electronics industry is so recent, many poten-
1. More payloald may be possible when using lithium tial users are not aware that lithium batteries are
batteries due to their smaller size and/or lighter not all alike. Lithium is only the first name of any
weight. lithium power source. Just as there are many zinc
2. More operating life will be achievable with the batteries available (zinc-carbon. zinc-silver oxide,
same size but lighter battery. zinc-mercuric oxide) there are many varieties of
3. Performance at colder temperatures will be pos- lithium system, each with its own peculiar internal
sible. chemistry and construction. Several of these systems
4. Longer active life will result in reduced main- are briefly described below.
tenance cost:; associated with battery replacements, Table 9.6 shows the lithium systems available for
especially in remote locations, or in other commercial use from Catalyst Research Corporation.
applications where replacing the battery is a labour- Most are button cells approximately 20-30mm in
intensive function. diameter and 1.5-3 mrn thick. All are high-voltage
5. Replacement inventory costs will be reduced cells with moderate or high internal resistances. The
because periodic replacement of batteries will be first two have seen use in watches or calculators
significantly decreased or eliminated. only; the remaining three have also been used in the
6. No special storage provisions are required. pacemaker field.
Like any common batteries, lithium batteries will
Even though cell costs may be high initially, peo- rupture if exposed to fire. The low-rate lithium
ple responsible for maximizing a system’s overall batteries, intended for watches, should be safe if used
cost effectiveness must look at the total life-cycle within manufacturers’ specified temperatures. Thick
tests and take these points into consideration. As separators in these low-rate cells prevent shorting
well as lithium--vanadium pentoxide, lithium-sulphur and their small size permits easy heat dissipation if
dioxide and lithium-thionyl chloride systems, there any local internal reactions should occur. In fact, a
exist other types of cell containing organic elec- good case can be made that most low-rate lithium
trolytes and lithium. For example, the lithium-copper cells are safer than zinc-mercury cells, whch can
fluoride couple has a theoretical voltage of 3.5V and introduce poisonous mercury into the atmosphere
a theoretical energy-to-weight ratio of 1575 W hkg. when incinerated. SAFT supply lithium-copper oxide
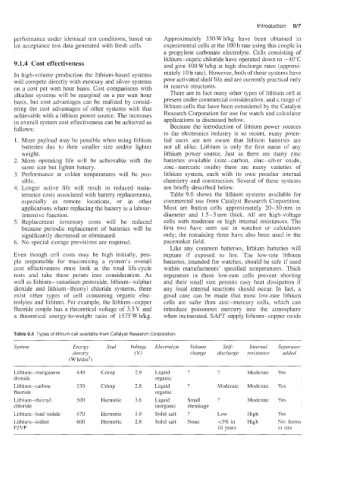
Table 9.8 Types of lithium cell available from Catalyst Research Corporation
System Energy Seal Voltage Electrolyte Volume Self- Internal Separator
density (VI change discharge resistance added
(Wh/dm3)
Lithium-manganese 440 Crimp 2.9 Liquid ? ? Moderate Yes
dioxide organic
Lithium-carbon 330 crimp 2.8 Liquid ? Moderate Moderate Yes
fluoride organic
Lithium-thionyl 500 Hermetic 3.6 Liquid Small ? Moderate Yes
chloride inorganic shnnkage
Lithium-lead iodide 470 Hermetic 1.9 Solid salt ? Low High Yes
in
Lithmm-iodine 600 Hermetic 2.8 Solid salt None ~5% High No: forms
P2VP 10 years in situ