Page 279 - Battery Reference Book
P. 279
Lithium-sulphur dioxide primary batteries 2413
Lithium-sulphur dioxide primary carbon electrode where the sulphur dioxide is reduced.
batteries Depending on the current density, the precipitate may
deposit throughout the porous cathode (at low current
The system uses a lithium anode, a gaseous sulphur densities) or predominantly at the surface of the cath-
dioxide cathode (about 70% of the weight of the ode facing the anode (at high current densities).
electrolyte depolai%zer) and an electrolyte comprising The nature of this discharge process shows that the
lithium bromide dissolved in acetonitrile. conductivity of the electrolyte plays an important role
The discharge at the anode may be represented by in determining the rate capability of the system, both
the half-cell reaction: from the point of view of the rate at which lithium ions
can be transported to the cathode and from the point
Li = Li+ + e- (24.1) of view of the depth to which the discharge reactio~
The cathode reaction may be written as: can penetrate into the cathode.
Figure 24.2 shows the composition-conductivity
2~02 + 2ep = ~202- (24.2) contour of the lithium bromide-sulphur dioxide-
acetonitrile electrolyte over a wide temperature range.
and the overail cell reaction as
A maximum conductivity of about 6 x 10p2/(G? cm) is
2Li + 2,502 = 12i2S20~(lithium dithionite) (24.3) obtained at 25°C. It may be seen that the conductivity
contours form a ridge which follows closely the sul-
The discharge of lithium-sulphur dioxide cells is phur dioxide depletion line. The latter is the extended
accompanied by the precipitation of lithium dithionite line connecting the sulphur dioxide apex in the diagram
in the cathodes, but the precipitate appears not to lessen and the point representing the initial composition of the
the rate capability of the cells noticeably. An optimal electrolyte used in the cells. To enhance the rate capa-
electrolyte composition can be specified that permits bility of the cells, an electrolyte composition should be
the cathodes to operate along a maximal conductivity selected on the sulphur dioxide-rich side of the con-
path during discharge. ductivity maximum and represented by the start of the
The high-rate capability of the cells is favoured by
the high conductivity of the electrolyte and the small arrow in Figure 24.2 (71-75% sulphur dioxide). This
choice is arbitrary to some extent, except for its loca-
effect of temp'erature on the conductivity. Heat dissipa- tion along the conductivity ridge. It was dictated by the
tion structures are recommended for lithium- sulphur recognition that, under the dynamic conditions of dis-
dioxide cells and batteries that operate at high power charge, sulphur dioxide depletion will occur inside the
levels. Lithium- sulphur dioxide cells can operate at porous cathode. Thus, by choosing a sulphur dioxide-
460 W/kg while delivering LOO Wkg in suitable high- rich electrolyte, depletion of the sulphur dioxide in the
rate configurations. cathode will in fact lead to an increased conductivity oi
On the basis of postulated cell reactions, the dis- the electrolyte in the cathode and an enhanced partic-
charge process of the lithium-sulphur dioxide cell may ipation of the interior of the cathode in the discharge
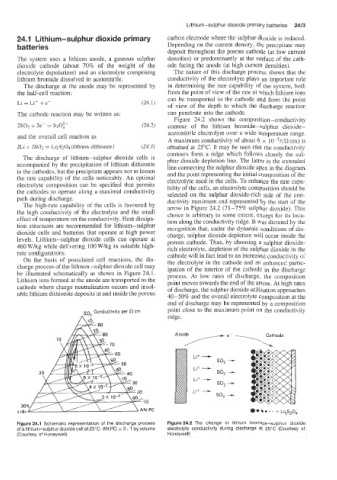
be illustrated schematically as shown in Figure 24.1. process. At low rates of discharge, the composition
Lithium ions formed at the anode are transported to the point moves towards the end of the arrow. At high rates
cathode where charge neutralization occurs and insol- of discharge, the sulphur dioxide utilization approaches
uble lithium dithionite deposits at and inside the porous 40-50% and the overall electrolyte composition at the
end of discharge may be represented by a cornposition
so Conductivity per s2 cm point close to the maximum point on the conductivity
ridge.
an
Li+ -
so, -+
Li+ -+
so, +
Lit d
so, --b
Li+ +
so, 4
10
30°
LiBr N:PC 0 0 0 * - . = Li,S20,
Figure 24.1 Schematic representation of the discharge process Figure 24.2 The change in lithium bromide-sulphur dioxide
of a lithium-sulphur dioxide cell at 25°C. AN:PC = 3 : 1 by volume electrolyte conductivity during discharge at 25°C (Courtesy of
(Courtesy of Honeywell) Honeywell)