Page 280 - Battery Reference Book
P. 280
24/4 Lithium batteries
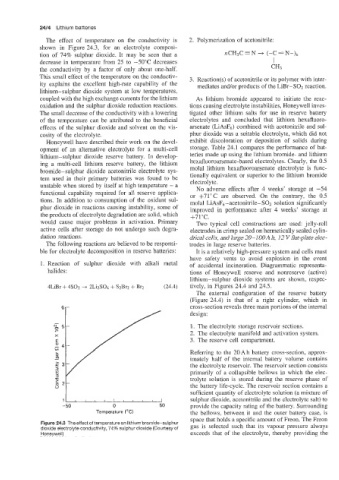
The effect of temperature on the conductivity is 2. Polymerization of acetonitrile:
shown in Figure 24.3, for an electrolyte composi-
3 (-C=N-),
tion of 74% sulphur dioxide. It may be seen that a PzCH~C=N
decrease in temperature from 25 to -50°C decreases I
the conductivity by a factor of only about one-half. CH3
This small effect of the temperature on the conductiv- 3. Reaction(s) of acetonitrile or its polymer with inter-
ity explains the excellent high-rate capability of the mediates and/or products of the LiBr-SO2 reaction.
lithium- sulphur dioxide system at low temperatures,
coupled with the high exchange currents for the lithium As lithium bromide appeared to initiate the reac-
oxidation and the sulphur dioxide reduction reactions. tions causing electrolyte instabilities, Honeywell inves-
The small decrease of the conductivity with a lowering tigated other lithium salts for use in reserve battery
of the temperature can be attributed to the beneficial electrolytes and concluded that lithium hexafluoro-
effects of the sulphur dioxide and solvent on the vis- arsenate (LiAsF6) combined with acetonitrile and sul-
cosity of the electrolyte. phur dioxide was a suitable electrolyte, which did not
Honeywell have described their work on the devel- exhibit discoloration or deposition of solids during
opment of an alternative electrolyte for a multi-cell storage. Table 24.1 compares the performance of bat-
lithium-sulphur dioxide reserve battery. In develop- teries made up using the lithium bromide- and lithium
ing a multi-cell lithium reserve battery, the lithium hexafluoroarsenate-based electrolytes. Clearly, the 0.5
bromide-sulphur dioxide acetonitrile electrolyte sys- molal lithium hexafluoroarsenate electrolyte is func-
tem used in their primary batteries was found to be tionally equivalent or superior to the lithium bromide
unstable when stored by itself at high temperature - a electrolyte.
No adverse effects after 4 weeks' storage at -54
functional capability required for all reserve applica- or +71"C are observed. On the contrary, the 0.5
tions. In addition to consumption of the oxidant sul- molal LiAsFd -acetonitrile-SOz solution significantly
phur dioxide in reactions causing instability, some of improved in performance after 4 weeks' storage at
the products of electrolyte degradation are solid, which +71"C.
would cause major problems in activation. Primary Two typical cell constructions are used: jelly-roll
active cells after storage do not undergo such degra- electrodes in crimp sealed on hermetically sealed cylin-
dation reactions. drical cells, and large 20-100Ah, 12V flat-plate elec-
The following reactions are believed to be responsi- trodes in large reserve batteries.
ble for electrolyte decomposition in reserve batteries: It is a relatively high-pressure system and cells must
have safety vents to avoid explosion in the event
1. Reaction of sulphur dioxide with alkali metal of accidental incineration. Diagrammatic representa-
halides: tions of Honeywell reserve and nonreserve (active)
lithium-sulphur dioxide systems are shown, respec-
4LiBr + 4so2 + 2Li2SO4 + S2Brz + Brz (24.4) tively, in Figures 24.4 and 24.5.
The external configuration of the reserve battery
(Figure 24.4) is that of a right cylinder, which in
6r cross-section reveals three main portions of the internal
design:
1. The electrolyte storage reservoir sections.
2. The electrolyte manifold and activation system.
3. The reserve cell compartment.
Referring to the 20 Ah battery cross-section, approx-
imately half of the internal battery volume contains
the electrolyte reservoir. The reservoir section consists
primarily of a collapsible bellows in which the elec-
trolyte solution is stored during the reserve phase of
the battery life-cycle. The reservoir section contains a
sufficient quantity of electrolyte solution (a mixture of
sulphur dioxide, acetonitrile and the electrolyte salt) to
- 50 0 50 provide the capacity rating of the battery. Surrounding
Temperature ("C) the bellows, between it and the outer battery case, is
space that holds a specific amount of Freon. The Freon
Figure 24.3 The effect of temperature on lithium bromide-sulphur gas is selected such that its vapour pressure always
dioxide electrolyte conductivity, 74% sulphur dioxide (Courtesy of
Honeywell) exceeds that of the electrolyte, thereby providing the