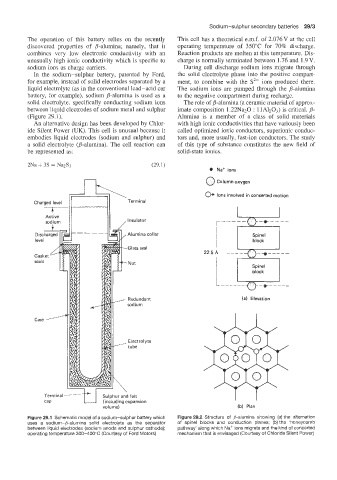
Page 324 - Battery Reference Book
P. 324
Sodium-sulphur secondary batteries 29/3
The operation of this battery relies on the recently This cell has a theoretical e.m.f. of 2.076 V at the cell
discovered properties of B-alumina; namely, that it operating temperature of 350°C for 70% discharge.
combines very low electronic conductivity with an Reaction products are molten at this temperature. Dis-
unusually high ionic conductivity which is specific to charge is normally terminated between 1.76 and 1.9 V.
sodium ions as charge carriers. During cell discharge sodium ions migrate through
In the sodium-sulphur battery, patented by Ford, the solid electrolyte phase into the positive compart-
for example, instead of solid electrodes separated by a ment, to combine with the S2- ions produced there.
liquid electrolyte (as in the conventional lead-acid car The sodium ions are pumped through the B-alumina
battery, for example), sodium B-alumina is used as a to the negative compartment during recharge.
solid electrolyte. specifically conducting sodium ions The role of B-alumina (a ceramic materid of approx-
between liquid electrodes of sodium metal and sulphur imate composition 1.22Na20 : llA1203) is critical. p-
(Figure 29.1). Alumina is a member of a class of solid materials
An alternative design has been developed by Chlor- with high ionic conductivities that have variousiy been
ide Silent Power (UK). This cell is unusual because it called optimized ionic conductors, superionic conduc-
embodies liquid electrodes (sodium and sulphur) and tors and, more usually. fast-ion conductors. The study
a solid electrolyte (p-alumina). The cell reaction can of this type of substance constitutes the new field of
be represented as: solid-state ionics.
2Na + 3s = Na2S3 (29.1)
0 Na+ ions
0 column oxygen
Ions involved in concerted motion
Charged level Terminal
7-
Active
sodium Insulator
Alumina collar
8-
Nut
Redundant
I (a) Elevation
sodium
Electrolyte
,--tube
\
uding expansion
volume) (b) Plan
Figure 29.1 Schematic model of a sodium-sulphur battery which Figure 29.2 Structure of p-alumina showing (a) the alternation
uses a sodium-p-alumina solid electrolyte as the separator of spinel blocks and conduction planes; (b) the 'honeycomb
between liquid electrodes (sodium anode and sulphur cathode), pathway' along which Na+ ions migrate and the kind of concerted
operating temperature 300-400°C (Courtesy of Ford Motors) mechanism that is envisaged (Courtesy of Chloride Silent Power)