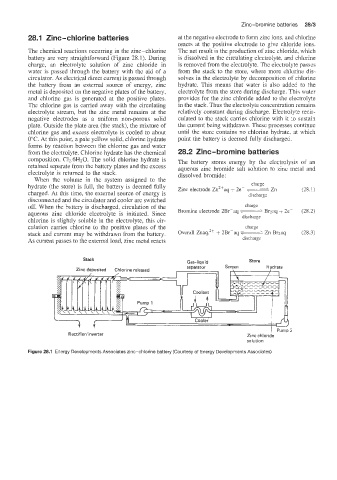
Page 320 - Battery Reference Book
P. 320
Zinc-bromine batteries 2
inc-chlorine batteries at the negative electrode to form zinc ions, and chlorine
reacts at the positive electrode to give chloride ions.
The chemical reactions occumng in the zinc-chlorine The net result is the production of zinc chloride, which
battery are very :straightforward (Figure 28.1). During is dissolved in the circulating electroiyte, and chlorine
charge, an electrolyte solution of zinc chloride in is removed from the electrolyte. The electrolyte passes
water is passed through the battery with the aid of a from the stack to the store, where more chlorine dis-
circuiator. As electrical direct current is passed through solves in the electrolyte by decomposition of chlorine
the battery from an external source of energy, zinc hydrate. This means that water is also added to the
metal is deposited on the negative plates of the battery, electrolyte from the store during discharge. This water
and chlorine gas is generated at the positive plates. provides for the zinc chloride added to the electrolyte
The chlorine gas is carried away with the circulating in the stack. Thus the electrolyte concentration remains
electrolyte stream, but the zinc metal remains at the relatively constant during discharge. Electrolyte recir-
negative electrodes as a uniform non-porous solid culated to the stack carries chlorine with it to sustain
plate. Outside the plate area (the stack), the mixture of the current being withdrawn. These processes continue
chlorine gas and excess electrolyte is cooled to about until the store contains no chlorine hydrate, at which
0°C. At this point, a pale yellow solid. chlorine hydrate point the battery is deemed fully discharged.
forms by reaction between the chlorine gas and water
from the electrolyte. Chlorine hydrate has the chemical 28.2 Zinc-bromine batteries
composition, C12 6Hz0. The solid chlorine hydrate is The battery stores energy by the electrolysis of an
retained separate from the battery plates and the excess aqueous zinc bromide salt solution to zinc metal and
electrolyte is returned to the stack. dissolved bromide:
When the volume in the system assigned to the
charge
hydrate (the store) is full, the battery is deemed fully Zinc electrode Z@aq + 2eC __i Zn (28.1)
charged. At this time, the external source of energy is discharge
disconnected and the circulator and cooler are switched
off. When the battery is discharged, circulation of the charge
aqueous zinc chloride electrolyte is initiated. Since Bromine electrode 2Br-aq F=====+ Brzaq + 2eC (28.2)
discharge
chlorine is slightly soluble in the electrolyte, this cir-
culation carries chlorine to the positive plates of the charge
stack and current may be withdrawn from the battery. Overall Znaq.” + 2Br-aq Zn Brzaq (28.3)
As current passes to the external load, zinc metal reacts discharge
Stack Gas-liquid Store
separator Screen Hydrate
Zinc deDosited Chlorine released
2
Rectifieriinverter Zinc chloride
solution
Figure 28.1 Energy Developments Assoclates zinc-chlorine battery (Courtesy of Energy Developments Associates)