Page 36 - Battery Reference Book
P. 36
Activities of electrolyte solutions 1/21
unity, and thle activity coefficient is then also unity on
the basis of the chosen standard state.
Several methods have beein devised for the deter- 0.6
mination of activities; measurements of vapour pres-
sure, freezing point depression, etc., have been used to
determine departure from ideal behaviour, and hence 0 0.5
to evaluate activities. The vapour pressure method had m
been used particularly to obtain the activity of the sol- .-
0
L
3
vent in the following manner. r
-
P 0.4
Equation 11.49 is applicable to any solution, ideal 2
or non-ideal. provided only that the vapour behaves r
0
as an ideal gas; comparison of this with Equation 1.49 c
C
.-
shows that the activity of the solvent in a solution must .8 0.3
r
r
be proportional to the vapour pressure of the solvent 8
over a given solution. If a represents the activity of c
>
.-
the solvent in the solution and p is its vapour pressure, 'S 0.2
c
then a = kp, where k is a proportionality constant. The 4
value of this constant can be determined by making
use of the standard state postulated above, namely 0.1
that a = 1 for the pure solvent, Le. when the vapour
pressure is po; it follows, therefore, that k, which is
equal to alp, is Upo, and hence
0 1 2 3 4 5
a=- P (1.57) Concentration of sulphuric acid (mollkg)
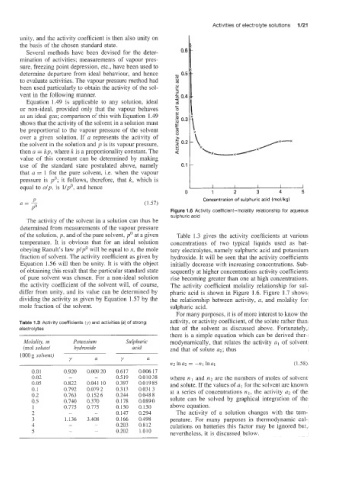
PO Figure 1.6 Activity coefficient-molality relationship for aqueous
sulphuric acid
The activity of the solvent in a solution can thus be
determined from measurements of the vapour pressure
of the solution, p, and of the pure solvent, po at a given Table 1.3 gives the activity coefficients at various
temperature. It is obvious that for an ideal solution concentrations of two typical liquids used as bat-
obeying Raonlt's law pIpo will be equal to x, the mole tery electrolytes, namely sulphuric acid and potassium
fraction of solvent. The activity coefficient as given by hydroxide. It will be seen that the activity coefficients
Equation 1.56 will then be unity. It is with the object initially decrease with increasing concentrations. Sub-
of obtaining this result that the particular standard state sequently at higher concentrations activity coefficients
of pure solvent was chosen. For a non-ideal solution rise becoming greater than one at high concentrations.
the activity coefficient of the solvent will, of course, The activity coefficient molality relationship for sul-
differ from unity, and its value can be determined by phuric acid is shown in Figure 1.6. Figure 1.7 shows
dividing the activity as given by Equation 1.57 by the the relationship between activity, a, and molality for
mole fraction of the solvent. sulphuric acid.
For many purposes, it is of more interest to know the
Table 1.3 Activity coefficients (y) and activities (a) of strong activity, or activity coefficient, of the solute rather than
electrolytes that of the solvent as discussed above. Fortunately,
there is a simple equation which can be derived ther-
Molality, in Potassium Sulphuric modynamically, that relates the activity al of solvent
(mol solute1 hydroxide acid and that of solute az; thus
1000 g solvent)
Y a Y a
n21naz = -nl lnal (1.58)
0.01 0.920 0.009 20 0.617 0.006 17
0.02 - - 0.519 0.010 38 where nl and n2 are the numbers of moles of solvent
0.05 0.822 0.041 10 0.397 0.019 85 and solute. If the values of al for the solvent are known
0.1 0.792 0.079 2 0.313 0.031 3 at a series of concentrations nl, the activity a2 of the
0.2 0.763 0.1526 0.244 0.048 8 solute can be solved by graphical integration of the
0.5 0.740 0.370 0.178 0.089 0
1 0.775 0.775 0.150 0.150 above equation.
2 - - 0.147 0.294 The activity of a solution changes with the tem-
3 1.136 3.408 0.166 0.498 perature. For many purposes in thermodynamic cal-
4 - - 0.203 0.812 culations on batteries this factor may be ignored but,
5 - - 0.202 1.010 nevertheless, it is discussed below.