Page 71 - Battery Reference Book
P. 71
1/56 Introduction to battery technology
From Equation 1.86, the calories generated per sec- 100
ond during the passage of a current of Z amps through
a resistance of R L2 is given by
90
12R
4.18 calls 80
~
s
Therefore, calories generated per second along con- -
ducting path AB (Figure 1.55) is a
Q 70
5
@ = ~ I2 ( -I2Rl (1.107) 2.
R2
4.18 4.18 R1 +R2 2 60
m
+
and calories generated per second along conducting 't 50
path AD is -
2
0)
(1.108) - 40
m
P
-
Consequently: Sj 30
0)
w
ca!./s along path AB - R2 0
-
-
cab along path AD R1 (1.109) = 20
Using Equations 1.107 and 1.108 it is possible to 10
calculate the calories per second generated along the
two paths for any particular values of R1, R2 and
current (I). 0
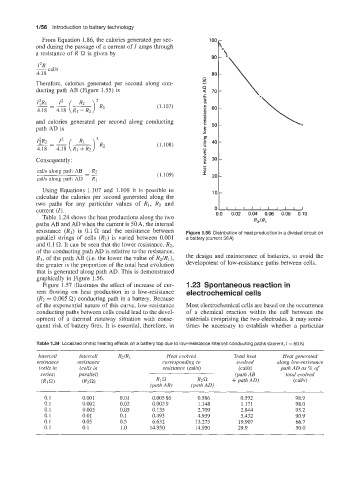
Table 1.24 shows the heat productions along the two 0.0 0.02 0.04 0.06 0.08 0.10
paths AB and AD when the current is 50 A, the internal RZIR,
resistance (R1) is 0.1 '2 and the resistance between Figure 1.56 Distribution of heat production in a divided circuit on
parallel strings of cells (R2) is varied between 0.001 a battery (current 50A)
and 0.1 a. It can be seen that the lower resistance, R2,
of the conducting path AD is relative to the resistance,
R1, of the path AB (Le. the lower the value of R2/R1), the design and maintenance of batteries, to avoid the
the greater is the proportion of the total heat evolution development of low-resistance paths between cells.
that is generated along path AD. This is demonstrated
graphically in Figure 1.56.
Figure 1.57 illustrates the effect of increase of cur- 1.23 Spontaneous reaction in
rent flowing on heat production in a low-resistance electrochemical cells
(Rz = 0.005 L2) conducting path in a battery. Because
of the exponential nature of this curve, low-resistance Most electrochemical cells are based on the occurrence
conducting paths between cells could lead to the devel- of a chemical reaction within the cell between the
opment of a thermal runaway situation with conse- materials comprising the two electrodes. It may some-
quent risk of battery fires. It is essential, therefore, in times be necessary to establish whether a particular
Table 1.24 Localized ohmic heating effects on a battery top due to low-resistance intercell conducting paths (current, I = 50A)
Intercell Intercell RdRi Heat evolved Total heat Heat generated
resistance resistance corresponding to evolved along low-resistance
(cells in (cells in resistance (calls) (cavs) path AD as % of
series) parallel) (path AB total evolved
(Ri Q) (R2Q) R1 Q R2 Q + path AD) (calls)
(path AB) (path AD)
0.1 0.001 0.01 0.005 86 0.586 0.592 98.9
0.1 0.002 0.02 0.002 9 1.148 1.171 98.0
0.1 0.005 0.05 0.135 2.709 2.844 95.2
0.1 0.01 0.1 0.493 4.939 5.432 90.9
0.1 0.05 0.5 6.632 13.275 19.907 66.7
0.1 0.1 1 .o 14.950 14.950 29.9 50.0