Page 72 - Battery Reference Book
P. 72
Spontaneous reaction in electrochemical cells 1/57
1100 hence,
0.OCl5 S2 (path AD) + +
.
.
AF = (~FL mFM + . . .) - (UFA + ~FB .) (1.110)
1 Do0
The molar free energy, F, of a substance in any state
can be expressed in terms of its activity, a, in that state
900 by means of Equation 1.1 10. which may be written as
F = FO +RT~"~ (1.111)
800
where Fo is the molar free energy in the standard state
700 of unit activity.
I If the values of FA, Fg, . . . , FL, FM, . . . , in
m
> Equation 1.1 10 are replaced by the corresponding
2 600 expressions derived from Equation 1.111, it is
P seen that
-
2 505 +
c AF = (l(F: + RTlnaL) + m(FL + RTI~uM) . . .)
I
I (U(F1 fRTIllaA)+b(Fi +RThUB)+...)
400
(1.112)
300 where UA, UB, . . . , aL, UM, are the activities of the
various substances involved in the reaction in their
arbitrary state. Upon rearranging Equation I. 1 12, the
200 result is
a; x ag x . . .
150 0.1 S2 (path AB) 4F = AFo +RTln (1.113)
S2
0.1
(path
...
a~xu~x
.¶ .¶ where AFO, the increase in free energy accompanying
0 200 400 800 800 1000 the reaction when all the reactants and products are in
200
800 1000
800
400
Current EA)
their respective standard states, is given by an equation
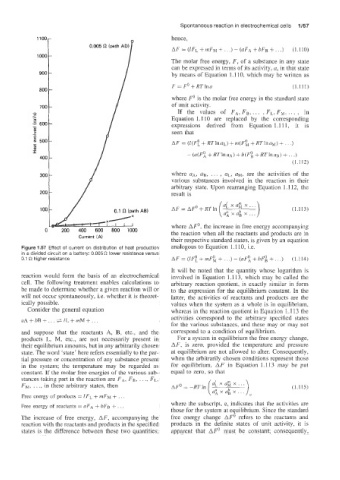
Figure 1.57 Eflect of current on distribution of heat production analogous to Equation 1.110, i.e.
in a divided circuit on a battery: 0.005 Q lower resistance versus
0.1 s2 higher resistance AF = (IF! + mFk + . . .) - (aFi + bFi + . . .) (l.il4)
It will be noted that the quantity whose logarithm is
reaction would form the basis of an electrochemical involved in Equation 1.113, which may be called the
cell. The following treatment enables calculations to arbitrary reaction quotient, is exactly similar in form
be made to dletermine whether a given reaction will or to the expression for the equilibrium constant. In the
will not occur spontaneously, i.e. whether it is theoret- latter, the activities of reactants and products are the
ically possible. values when the system as a whole is in equilibrium,
Consider the general equation whereas in the reaction quotient in Equation I. 1 I3 the
aA + bB +. . . + lL+mM+ . . . activities correspond to the arbitrary specified states
for the various substances, and these may or may not
and suppose that the reactants A, B, etc., and the correspond to a condition of equilibrium.
products L, M. etc., are not necessarily present in For a system in equilibrium the free energy change,
their equilibrium amounts, but in any arbitrarily chosen A F, is zero, provided the temperature and pressure
state. The word 'state' here refers essentially to the par- at equilibrium are not allowed to alter. Consequently,
tial pressure or concentration of any substance present when the arbitrarily chosen conditions represent those
in the system; the temperature may be regarded as for equilibrium, AF in Equation 1.113 may be put
constant. If the molar free energies of the various sub- equal to zero, so that
stances taking part in the reaction are FA, FB, . . ., FL,
FM, . . ., in these arbitrary states, then 4F0 = -RTln a[ xu$ x ... (1.115)
Free energy o'f products = ~FL + mFM + ai xa;x ...
+
Free energy of reactants = aFA + ~FB . . where the subscript, e, indicates that the activities are
.
those for the system at equilibrium. Since the standard
The increase of free energy, AF, accompanying the free energy change AFo refers to the reactants and
reaction with the reactants and products in the specified products in the definite states of unit activity, it is
states is the difference between these two quantities; apparent that AFo must be constant; consequently,