Page 69 - Battery Reference Book
P. 69
1/54 Introduction to battery technology
0.4 -
-
rn
1
0.4 Z 0.3 -
- - 0.028
Y
0 - 0.026
P
- c - 0.024 I
Ln (0 .- - 0.022 ::
I U
Y - .- - - 0.020 2
Fi 0.2
p 0.2
m L -
F 0 0.018
3
r P - 0.016
M .- - -
0 P - 0.014
.- P 0.2 0.012 .g
L . - 0.010 4
a z 0.1
U r -
P - 0.008 tu
-
0 - 0.006
0.004
0.1 - 0.002
I I I I I I I I ) -0
0 100 200 300 400 500 600 700
Discharge current (A)
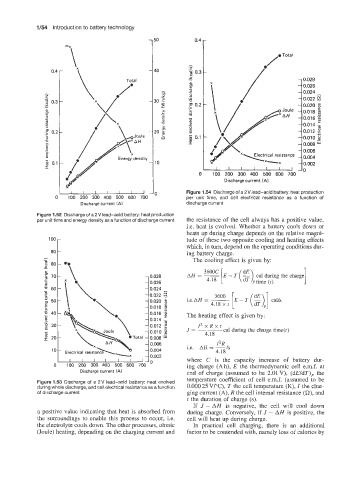
I I I I I I I Figure I .54 Discharge of a 2 V lead-acid battery: heat production
c 100 200 300 400 500 600 70( per unit time, and cell electrical resistance as a function of
Discharge current (A) discharge current
Figure 1.52 Discharge of a 2 V lead-acid battery: heat production
per unit time and energy density as a function of discharge current the resistance of the cell always has a positive value,
i.e. heat is evolved. Whether a battery cools down or
heats up during charge depends on the relative magni-
'OoC tude of these two opposite cooling and heating effects
which, in turn, depend on the operating conditions dur-
90
ing battery charge.
The cooling effect is given by:
1
- 0.028 cal during the charge
- 0.026 4.18
- 0.024 -
- 0.022 c:
- 0.020 E
'D 50-
0.018 $
0.016 '5 The heating effect is given by:
0.014
0.012 12xRxt
J=- cal during the charge time@)
Y 4.18
I~R
0.006 Le. AH = -Is
lo - Electrical resistadLx - 0.004 4.18
-
0.002
I I I I I I I o where C is the capacity increase of battery dur-
0 100 200 300 400 500 600 700 ing charge (Ah), E the thermodynamic cell e.m.f. at
Discharge current (A) end of charge (assumed to be 2.01V), (dEldT), the
temperature coefficient of cell e.m.f. (assumed to be
Figure 1.53 Discharge of a 2V lead-acid battery: heat evolved
during whole discharge, and cell electrical resistance as a function 0.000 25 VPC), T the cell temperature (K), I the char-
of discharge current ging current (A), R the cell internal resistance (a), and
t the duration of charge (s).
If J - AH is negative, the cell will cool down
a positive value indicating that heat is absorbed from during charge. Conversely, if J - AH is positive, the
the surroundings to enable this process to occur, i.e. cell will heat up during charge.
the electrolyte cools down. The other processes, ohmic In practical cell charging, there is an additional
(Joule) heating, depending on the charging current and factor to be contended with, namely loss of calories by