Page 78 - Battery Reference Book
P. 78
Pressure development in sealed batteries 3/63
Waving ascertained ia/ic from the known values of
E, and E,, assume that i, has a value of 10 A, Le.
10
i --
c-.
za&
(Table 1.21). From the anode reaction:
PbO2 + 2H' i- 2e + SO?- = PbSo4 + H2O + $02
2 x 96 500 C liberate 11 200 cm3 oxygen, i.e. a current
of 10 A for 1 s liberates
10 x 11 200 =Z 0.5803 cm3 oxygeds
2 x 96500
From the cathode reaction:
PbSO4 + 2Hf = PbS04 + H2
position (33.3% v/
2 x 96 500 C liberate 22 400 cm3 hydrogen, i.e. a cur- - - - - - - -
rent of 101(i8/ic) A for 1 s liberates
10 x 22400 lOi,
(ia/i,.) x 2 x 96500 = ~ i, x 0.1 160 cm3 hydrogeds
From these data, it is possible, as shown in Table
1.25, to calculate the volume of oxygen produced
per second at the anode and the volume of hydrogen
produced per second at the cathode, hence the total
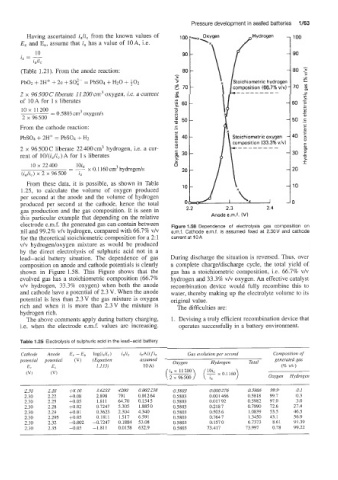
gas production and the gas composition. IC is seen in 2.2 2.3 2.4
this particular example that depending on the relative Anode e.m.f. (V)
electrode e.m.f. the generated gas can contain between Figure 1.58 Dependence of electrolysis gas composition on
nil and 99.2% vlv hydrogen, compared with 66.7% vlv e.m.f. Cathode e.m.f. is assumed fixed at 2.30V and cathode
for the theoretical stoichiometric composition for a 2: 1 current at 10 A
vlv hydrogeldoxygen mixture as would be produced
by the direct electrolysis of sulphuric acid not in a
lead-acid battery situation. The dependence of gas During discharge the situation is reversed. Thus, over
composition on anode and cathode potentials is clearly a complete charge/discharge cycle, the total yield of
shown in Figure 1.58. This Figure shows that the gas has a stoichiometric composition, i.e. 66.7% v/v
evolved gas has a stoichiometric composition (66.7% hydrogen and 33.3% vlv oxygen. An effective catalyst
v/v hydrogen, 33.3% oxygen) when both the anode recombination device would fully recombine this to
and cathode have a potential of 2.3 V. When the anode water, thereby making up the electrolyte volume to its
potential is less than 2.3V the gas mixture is oxygen original value.
rich and when it is more than 2.3V the mixture is The difficulties are:
hydrogen rich.
The above comments apply during battery charging, 1. Devising a truly efficient recombination device that
i.e. when the electrode e.m.f. values are increasing. operates successfully in a battery environment.
Table 1.25 Elesctrolysis of sulphuric acid in the lead-acid battery
Cathode Anode log (i& ) iJi, i,A(i f i, Gas evolution per second Composition of
potential potential (Equation assumed Oxygen Hydrogen Total generated gas
E, E, 1.133) 10A) (% vh)
(VI (VI
Oxygen Hydrogen
2.30 2.20 +0.10 3.6233 4200 0.002238 0.5803 0.000 276 0.5806 99.9 0.1
2.30 2.22 +0.08 2.898 791 0.01264 0.5803 0.001 466 0.5818 99.7 0.3
2.30 2.25 +0.05 1.811 64.70 0.1545 0.5803 0.017 92 0.5982 97.0 3.0
2.30 2.28 1-0.02 0.7247 5.305 1.8850 0.5803 0.2187 0.7990 72.6 27.4
2.30 2.29 +0.01 0.3623 2.304 4.340 0.5803 0.503 6 1.0839 53.5 46.5
2.30 2.295 +0.05 0.1811 1.517 6.591 0.5803 0.764 7 1.3450 43.1 56.9
2.30 2.32 -0.002 -0.7247 0.1884 53.08 0.5803 0.1570 6.7373 8.61 91.39
2.30 2.35 -0.05 -1.811 0.0158 632.9 0.5803 73.417 73.997 0.78 99.22