Page 83 - Battery Reference Book
P. 83
W4 Guidelines to battery selection
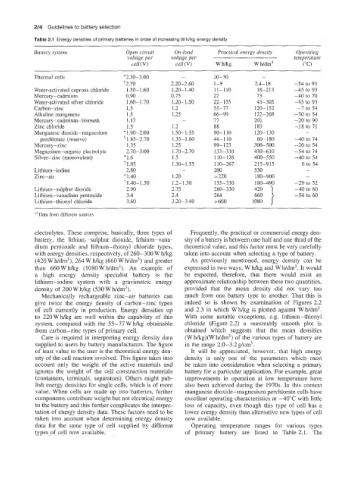
Table 2.1 Energy densities of primary batteries in order of increasing W h/kg energy density
Battery system Open circuit On-load Practical energy dens& Operating
voltage per voltage per temperature
cell (V) cell (V) wmg w wdm3 (" C)
Thermal cells *2.30-3.00 - 10-30 -
+2.70 2.20-2.60 1-9 2.4-18 -54 to 93
Water-activated cuprous chloride 1.50-1.60 1.20- 1.40 11-110 18-213 -45 to 93
Mercury-cadmium 0.90 0.75 22 73 -40 to 70
Water-activated silver chloride 1.60-1.70 1.20- 1 so 22-155 43-305 -45 to 93
Carbon-zinc 1.5 1.2 55-77 120-152 -1 to 54
Alkaline manganese 1.5 1.25 66-99 122-268 -30 to 54
Mercury- cadmium-bismuth 1.17 - 17 201 -20 to 90
Zinc chloride 1.5 1.2 88 183 -18 to 71
Manganese dioxide-magnesium * 1.90-2.00 1.50- 1.55 90-110 120-130 -
perchlorate (reserve) 1.85-2.70 1.35- 1.60 44-110 60-180 -40 to 74
Mercury -zinc 1.35 1.25 99-123 300-500 -20 to 54
Magnesium-organic electrolyte 2.70-3.00 1.70-2.70 133-330 430-610 -54 to 74
Silver-zinc (monovalent) *1.6 1.5 110-126 400-550 -40 to 54
+ 1.85 1.30-1.55 110-267 215-915 0 to 54
Lithium-iodine 2.80 - 200 530 -
Zinc-air * 1.40 1.20 > 220 180-900 -
1.40- 1.50 1.2- 1.30 155-330 180-490 -29 to 52
420
Lithium-sulphur dioxide 2.90 2.15 260-330 660 } -40 to 60
Lithium-vanadium pentoxide 3.4 2.4 264 -54 to 60
Lithium-thionyl chloride 3.60 3.20-3.40 2660 1080
"+Data from different sources
electrolytes. These comprise, basically, three types of Frequently, the practical or commercial energy den-
battery, the lithiun; -sulphur dioxide, lithium-vana- sity of a battery is between one-half and one-third of the
dium pentoxide and lithium-thionyl chloride types, theoretical value, and this factor must be very carefully
with energy densities, respectively, of 260-300 W h/kg taken into account when selecting a type of battery.
(420 W h/dm3), 264 W h/kg (660 W h/dm3) and greater As previously mentioned, energy density can be
than 660Whkg (1080Wh/dm3). An example of expressed in two ways; W hkg and W h/dm3. It would
a high energy density specialist battery is the be expected, therefore, that there would exist an
lithium-iodine system with a gravimetric energy approximate relationship between these two quantities,
density of 200 W h/kg (530 W h/dm3). provided that the mean density did not vary too
Mechanically rechargeable zinc-air batteries can much from one battery type to another. That this is
give twice the energy density of carbon-zinc types indeed so is shown by examination of Figures 2.2
of cell currently in production. Energy densities up and 2.3 in which Whkg is plotted against Wh/dm3.
to 220Whkg are well within the capability of this With some notable exceptions, e.g. lithium-thionyl
system, compared with the 55-77 W h/kg obtainable chloride (Figure 2.2) a reasonably smooth plot is
from carbon-zinc types of primary cell. obtained which suggests that the mean densities
Care is required in interpreting energy density data (W hkg)(W h/dm3) of the various types of battery are
supplied to users by battery manufacturers. The figure in the range 2.0-3.2g/cm3.
of least value to the user is the theoretical energy den- It will be appreciated, however, that high energy
sity of the cell reaction involved. This figure takes into density is only one of the parameters which must
account only the weight of the active materials and be taken into consideration when selecting a primary
ignores the weight of the cell construction materials battery for a particular application. For example, great
(containers, terminals, separators). Others might pub- improvements in operation at low temperature have
lish energy densities for single cells, which is of more also been achieved during the 1970s. In this context
value. When cells are made up into batteries, further manganese dioxide-magnesium perchlorate cells have
components contribute weight but not electrical energy excellent operating characteristics at -40°C with little
to the battery and this further complicates the interpre- loss of capacity, even though this type of cell has a
tation of energy density data. These factors need to he lower energy density than alternative new types of cell
taken into account when determining energy density now available.
data for the same type of cell supplied by different Operating temperature ranges for various types
types of cell now available. of primary battery are listed in Table 2.1. The