Page 82 - Battery Reference Book
P. 82
Primary batteries 2/13
The first choice to be made is whether a primary densities, the open circuit and on-load cell emf. and
or secondaq batteq is required. Usually, there is no the minimum and maximum recommended operating
doubt about the requirement in this respect. We shall temperatures of a wide range of primary batteries.
therefore pnoceed to a discussion of the selection of These are some, but not all, OS the factors that must be
the particulin type of primary or secondary battery taken into account when selecting a type of battery.
required for the application in mind. Gravimetric energy density controls the weight of
There is increasing environmental pressure to stop the battery required for a given energy output. Primary
the use of mercury batteries. As these are still used carbon-zinc and alkaline manganese dioxide batteries
to a limited extent, eg. some military applications, a have relatively low gravimetric energy densities in
discussion of them is included in this book. the ranges, respectively, of 55-77 and 66-99Whkg
or 120-152 and 122-268Wh/dm3 on a volumetric
basis. Only mercury-cadmium batteries and specialist
2.1 Primary batteries batteries developed for particular applications, such
Until the 1970s, primary batteries were predom- as the thermally activated batteries and cuprous
inantly zinc-anode-based systems. Performance of chloride and silver chloride type seawater-activated
these cells has undergone progressive improvements batteries, have lower gravimetric energy densities
through development of the original Leclanch6 than the carbon-zinc and alkaline manganese dioxide
(carbon-zinc) system and introduction of new types. More recently developed primary batteries, such
couples such as zinc-mercuric oxide, alkaline as the manganese dioxide-magnesium perchlorate
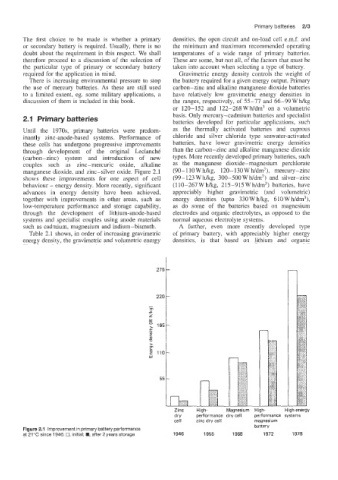
manganese (dioxide, and zinc-silver oxide. Figure 2.1 (90- 1 10 W h/kg, 120- 130 W h/dm3), mercury -zinc
shows these improvements for one aspect of cell (99- 123 W hkg, 300-500 W h/dm3) and silver-zinc
behaviour - energy density. More recently, significant (110-267 Whkg, 215-915 Wh/dm3) batteries, have
advances in energy density have been achieved, appreciably higher gravimetric (and volumetric)
together with improvements in other areas, such as energy densities (upto 330 W hkg, 610 W h/dm3),
low-temperature performance and storage capability, as do some of the batteries based on magnesium
through the development of lithium-anode-based electrodes and organic electrolytes, as opposed to the
systems and specialist couples using anode materials normal aqueous electrolyte systems.
such as cadmium, magnesium and indium-bismuth. A further, even more recently developed type
Table 2.1 shows, in order of increasing gravimetric of primary battery, with appreciably higher energy
energy density, the gravimetric and volumetric energy densities, is that based on lithium and organic
275 -
220 -
.
..-..
m
Y
L
2
~ 165-
._
c
E
-0
1
P
E 110-
w
Zinc High- Magnesium High- High-energy
dry performance dry cell performance systems
cell zinc dry cell magnesium
battery
Figure 2.1 lrriprovement in primary battery performance
at 21 "C since 1946: 0, initial: W, after 2 years storage 1946 1955 1968 1972 1978