Page 84 - Battery Reference Book
P. 84
Primary batteries 2/5
1100-
/
/
1000 / / 1000 -
/
/'
900 / /
/
/
800 / /
"E . 7001
U
1
I '
; 600'
._
h 5001
E?
C
Lu
40#
300
12
0 100 200 300 400 500 600 700 0 100 200 300 400 500 600 700
Energy density (W h/kg) Energy densiry (W h/kg)
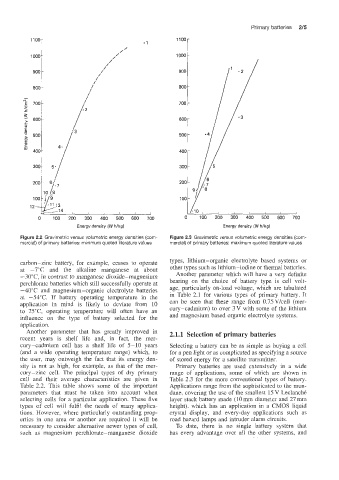
Figure 2.2 Gravimetric versus volumetric energy densities (com- Figure 2.3 Gravimetric versus volumetric energy densities (com-
mercial) of primary batteries: minimum quoted literature values mercial) of primary batteries: maximum quoted literature values
carbon-zinc battery, for example, ceases to operate types, lithium-organic electrolyte based systems or
at -7°C arid the alkaline manganese at about other types such as lithium-iodine or thermal batteries.
-3O"C, in contrast to manganese dioxide-magnesium Another parameter which will have a very definite
perchlorate batteries which still successfully operate at bearing on the choice of battery type is cell volt-
-40°C and magnesium-organic electrolyte batteries age, particularly on-load voltage, which are tabulated
at -54°C. If battery operating temperature in the in Table 2.1 for various types of primary battery. It
application in mind is likely to deviate from 10 can be seen that these range from 0.75Vkell (mer-
to 25T, operating temperature will often have an cury-cadmium) to over 3 V with some of the lithium
influence on the type of battery selected for the and magnesium based organic electrolyte systems.
application.
Another parameter that has greatly improved in 2.1.1 Selection of primary batteries
recent years is shelf life and, in fact, the mer-
cury-cadmium cell has a shelf life of 5-10 years Selecting a battery can be as simple as buying a cell
(and a wide operating temperature range) which, to for a pen light or as complicated as specifying a source
the user. may outweigh the fact that its energy den- of stored energy for a satellite transmitter.
sity is not as high, for example, as that of the mer- Primary batteries are used extensively in a wide
cury-zinc cell. The principal types of dry primary range of applications, some of which are shown in
cell and their average characteristics are given in Table 2.3 for the more conventional types of battery.
Table 2.2. This table shows some of the important Applications range from the sophisticated to the mun-
parameters that must be taken into account when dane, covering the use of the smallest 15 V LeclanchC
selecting cells for a particular application. These five layer stack battery made (10 mm diameter and 27 mm
types of cell will fulfil the needs of many applica- height), which has an application in a CMOS liquid
tions. However, where particularly outstanding prop- crystal display, and everyday applications such as
erties in one area or another are required it will be road hazard lamps and intruder alarm circuits.
necessary to consider alternative newer types of cell, To date, there is no single battery system that
such as magnesium perchlorate-manganese dioxide has every advantage over all the other systems, and