Page 96 - Battery Reference Book
P. 96
2/14 Guidelines to battery selection
discharged to 1.0V may be brought to the fully
charged state in 12 h by charging at the C8 rate. The
nickel-cadmium system will accept overcharge more
readily than the lead-acid battery, but for permanent
trickle charging a lower charge current than the above
is recommended.
2.2 Secondary batteries
Table 2.8 shows, in order of increasing gravimet-
ric density, the gravimetric and volumetric densities,
the open circuit and on-load cell e.m.f. values and
5
0 20 40 60 80 100 the minimum and maximum recommended operat-
Percentage of service life ing temperatures of a range of secondary batter-
ies. For simplicity, Table 2.8 tabulates the mean data
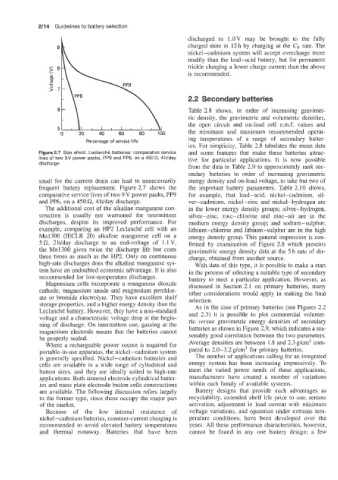
Figure 2.7 Size effect. Leclanche batteries: comparative service and some features that make these batteries attrac-
lives of two 9V power packs, PP9 and PP6, on a 450Q, 4 h/day tive for particular applications. It is now possible
discharge
from the data in Table 2.9 to approximately rank sec-
ondary batteries in order of increasing gravimetric
small for the current drain can lead to unnecessarily energy density and on-load voltage, to take but two of
frequent battery replacement. Figure 2.7 shows the the important battery parameters. Table 2.10 shows,
comparative service lives of two 9 V power packs, PP9 for example, that lead-acid, nickel-cadmium, sil-
and PP6, on a 450 Q, 4 Wday discharge. ver-cadmium, nickel-zinc and nickel-hydrogen are
The additional cost of the alkaline manganese con- in the lower energy density groups; silver-hydrogen,
struction is usually not warranted for intermittent silver-zinc, zinc-chlorine and zinc-air are in the
discharges, despite its improved performance. For medium energy density group; and sodium-sulphur,
example, comparing an HP2 LeclanchC cell with an lithium-chlorine and lithium-sulphur are in the high
Mn1300 (IECLR 20) alkaline manganese cell on a energy density group. This general impression is con-
5 C2, 2hlday discharge to an end-voltage of 1.1 V, firmed by examination of Figure 2.8 which presents
the Mn1300 gives twice the discharge life but costs gravimetric energy density data at the 5 h rate of dis-
three times as much as the HP2. Only on continuous charge, obtained from another source.
high-rate discharges does the alkaline manganese sys- With data of this type, it is possible to make a start
tem have an undoubted economic advantage. It is also in the process of selecting a suitable type of secondary
recommended for low-temperature discharges. battery to meet a particular application. However, as
Magnesium cells incorporate a manganese dioxide discussed in Section 2.1 on primary batteries, many
cathode, magnesium anode and magnesium perchlor- other considerations would apply in making the final
ate or bromide electrolyte. They have excellent shelf selection.
storage properties, and a higher energy density than the As in the case of primary batteries (see Figures 2.2
LeclanchC battery. However, they have a non-standard and 2.3) it is possible to plot commercial volumet-
voltage and a characteristic voltage drop at the begin- ric veysus gravimetric energy densities of secondary
ning of discharge. On intermittent use, gassing at the batteries as shown in Figure 2.9, which indicates a rea-
magnesium electrode means that the batteries cannot
be properly sealed. sonably good correlation between the two parameters.
Where a rechargeable power source is required for Average densities are between 1.8 and 2.3 g/cm3 com-
portable-in-use apparatus, the nickel-cadmium system pared to 2.0-3.2 g/cm3 for primary batteries.
is generally specified. Nickel-cadmium batteries and The number of applications calling for an integrated
cells are available in a wide range of cylindrical and energy system has been increasing impressively. To
button sizes, and they are ideally suited to high-rate meet the varied power needs of these applications,
applications. Both sintered electrode cylindrical batter- manufacturers have created a number of variations
ies and mass plate electrode button cells constructions within each family of available systems.
are available. The following discussion refers largely Battery designs that provide such advantages as
to the former type, since these occupy the major part recyclability, extended shelf life prior to use, remote
of the market. activation, adjustment in load current with minimum
Because of the low internal resistance of voltage variations, and operation under extreme tem-
nickel-cadmium batteries, constant-current charging is perature conditions, have been developed over the
recommended to avoid elevated battery temperatures years. All these performance characteristics, however,
and thermal runaway. Batteries that have been cannot be found in any one battery design; a few