Page 100 - Battery Reference Book
P. 100
Secondary batteries 2/17
3CU
cn
Y
2
3 -
>
r
.z 20c
W
U -
> t
P
C W
w g -
>
v) .- z
- B
- -
L
m
Q
I
0
1 CIC E .-
._ N
2
E C
P e
I -
Y >:<::.:!
m
.- ......
........
........
..... :/.:
.+:.:..
>>>;
.....
...:.:.:.: >:(.??
.......... ..........
0..
..........
..........
..........
$$$;*
.......... :.:::::. (:::,:.::
.........
..........
.:.:.:.:.:
.........
..........
.>:.:.:.:
'..
ll B $$;g
:".
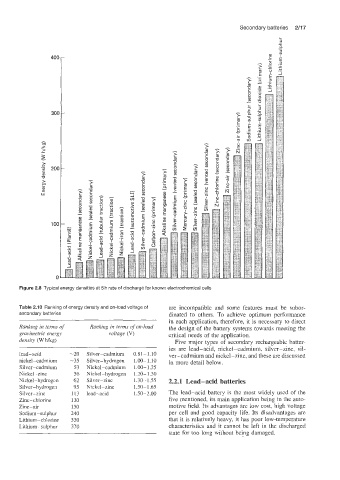
Figure 2.8 Typical energy densities at 5 h rate of discharge for known electrochemical cells
Table 2.10 Ranking of energy density and on-load voltage of are incompatible and some features must be subor-
secondary batteries dinated to others. To achieve optimum performance
in each application, therefore, it is necessary to direct
Ranking in terms of Ranking in terms of on-load the design of the battery systems towards meeting the
gravimetric energy voltage (V) critical needs of the application.
density (W Wkg) Five major types of secondary rechargeable batter-
ies are lead-acid, nickel-cadmium, silver-zinc, sil-
lead-acid -20 Silver-cadmium 0.81 - 1.10 ver-cadmium and nickel-zinc, and these are discussed
nickel-cadmium -35 Silver-hydrogen 1.00- 1.10 in more detail below.
Silver-cadmium 53 Nickel-cadmium 1.00-1.25
Nickel-zinc 56 Nickel-hydrogen 1.20-1.30
Nickel-hydrogen 62 Silver-zinc 1.30- 1.55 2.2.1 Lead-acid batteries
Silver- hydrogen 95 Nickel-zinc 1.50-1.65
Silver-zinc 113 lead-acid 1.50-2.00 The lead-acid battery is the most widely used of the
Zinc-chlorine 130 five mentioned, its main application being in the auto-
Zinc-air 150 motive field. Its advantages are low cost, high voltage
Sodium-sulphur 240 per cell and good capacity life. Its disadvantages are
Lithium-chlorine 330 that it is relatively heavy, it has poor low-temperature
Lithium -sulphur 370 characteristics and it cannot be left in the discharged
state for too long without being damaged.