Page 29 - Bebop to The Boolean Boogie An Unconventional Guide to Electronics Fundamentals, Components, and Processes
P. 29
10 Chapter Two
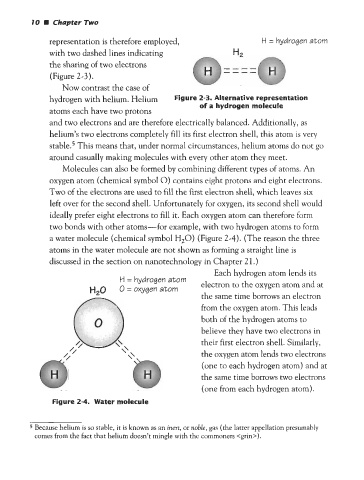
representation is therefore employed, H = hydrogen atom
with two dashed lines indicating H2
the sharing of two electrons ----
----
(Figure 2-3).
Now contrast the case of
hydrogen with helium. Helium Figure 2-3. Alternative representation
of a hydrogen molecule
atoms each have two protons
and two electrons and are therefore electrically balanced. Additionally, as
helium’s two electrons completely fill its first electron shell, this atom is very
stable.’ This means that, under normal circumstances, helium atoms do not go
around casually making molecules with every other atom they meet.
Molecules can also be formed by combining different types of atoms. An
oxygen atom (chemical symbol 0) contains eight protons and eight electrons.
Two of the electrons are used to fill the first electron shell, which leaves six
left over for the second shell. Unfortunately for oxygen, its second shell would
ideally prefer eight electrons to fill it. Each oxygen atom can therefore form
two bonds with other atoms-for example, with two hydrogen atoms to form
a water molecule (chemical symbol H,O) (Figure 2-4). (The reason the three
atoms in the water molecule are not shown as forming a straight line is
discussed in the section on nanotechnology in Chapter 21.)
Each hydrogen atom lends its
H = hydrogen atom
electron to the oxygen atom and at
the same time borrows an electron
from the oxygen atom. This leads
both of the hydrogen atoms to
believe they have two electrons in
their first electron shell. Similarly,
the oxygen atom lends two electrons
(one to each hydrogen atom) and at
the same time borrows two electrons
(one from each hydrogen atom).
Figure 2-4. Water molecule
5 Because helium is so stable, it is known as an inert, or noble, gas (the latter appellation presumably
comes from the fact that helium doesn’t mingle with the commoners <grin>).