Page 31 - Bebop to The Boolean Boogie An Unconventional Guide to Electronics Fundamentals, Components, and Processes
P. 31
Conductors and Insulators;
Voltage, Current, Resistance,
Capacitance, and Inductance
A substance that conducts electricity easily is called a conductor. Metals
such as copper are very good conductors because the bonds in their amorphous
crystalline structures are relatively weak, and the bonding electrons can easily
migrate from one atom to another. If a piece of copper wire is used to connect
a source with an excess of electrons to a target with too few electrons, the wire
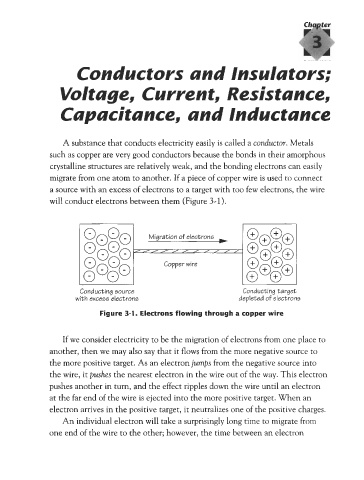
will conduct electrons between them (Figure 3-1).
Migration of electrons
Copper wire
Conducting source Conducting target
with excess electrons depleted of electrons
Figure 3-1. Electrons flowing through a copper wire
If we consider electricity to be the migration of electrons from one place to
another, then we may also say that it flows from the more negative source to
the more positive target. As an electron jumps from the negative source into
the wire, it pushes the nearest electron in the wire out of the way. This electron
pushes another in turn, and the effect ripples down the wire until an electron
at the far end of the wire is ejected into the more positive target. When an
electron arrives in the positive target, it neutralizes one of the positive charges.
An individual electron will take a surprisingly long time to migrate from
one end of the wire to the other; however, the time between an electron