Page 28 - Bebop to The Boolean Boogie An Unconventional Guide to Electronics Fundamentals, Components, and Processes
P. 28
Atoms, Molecules, and Crystals rn 9
bonds with other atoms. The atoms share each other's electrons, thereby
forming more complex structures. One such structure is called a molecule; for
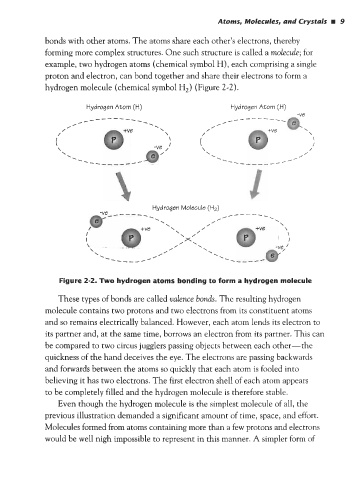
example, two hydrogen atoms (chemical symbol H), each comprising a single
proton and electron, can bond together and share their electrons to form a
hydrogen molecule (chemical symbol H,) (Figure 2-2).
Hydrogen Atom (H) Hydrogen Atom (H)
--_
_----_ . /------- -- -ve
/-- /
0
0 \ 0'
/ +ve \ / +ve \
\ \ \ \
'. I I
\ \ \ -. .
/
/
--- --____--- \ ----__- 0
_--/
0 .------
Hydrogen Molecule (Hz) .
\ \
\ 0 \
\ /
/ +ve \
\ /
\/ \
I
/
'-. 0 0 \
0
Figure 2-2. Two hydrogen atoms bonding to form a hydrogen molecule
These types of bonds are called valence bonds. The resulting hydrogen
molecule contains two protons and two electrons from its constituent atoms
and so remains electrically balanced. However, each atom lends its electron to
its partner and, at the same time, borrows an electron from its partner. This can
be compared to two circus jugglers passing objects between each other-the
quickness of the hand deceives the eye. The electrons are passing backwards
and forwards between the atoms so quickly that each atom is fooled into
believing it has two electrons. The first electron shell of each atom appears
to be completely filled and the hydrogen molecule is therefore stable.
Even though the hydrogen molecule is the simplest molecule of all, the
previous illustration demanded a significant amount of time, space, and effort.
Molecules formed from atoms containing more than a few protons and electrons
would be well nigh impossible to represent in this manner. A simpler form of