Page 160 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 160
136 6 Chemo-Enzymatic Cascade Reactions for the Synthesis of Glycoconjugates
6.1.2.2 Glycosidases and Glycosynthases
Glycosidases are another major tool in the synthesis of glycoconjugates. Their native
activity can be shifted under appropriate conditions toward the synthesis of glyco-
conjugates rather than the hydrolysis of glycosidic bonds. Altering thermodynamic
(equilibrium-controlled synthesis) or kinetic conditions (kinetically controlled pro-
cesses), respectively, glycosidases catalyze reverse hydrolysis or transglycosylation
reactions [44, 45]. Transglycosylation is preferred in the setting of cascade reactions
(Scheme 6.2).
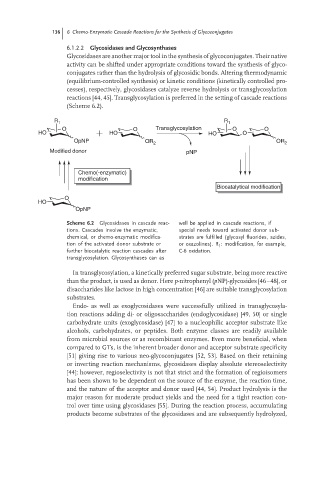
R 1 R 1
O O Transglycosylation O O
HO HO HO O
OpNP OR 2 OR 2
Modified donor pNP
Chemo(-enzymatic)
modification
Biocatalytical modification
O
HO
OpNP
Scheme 6.2 Glycosidases in cascade reac- well be applied in cascade reactions, if
tions. Cascades involve the enzymatic, special needs toward activated donor sub-
chemical, or chemo-enzymatic modifica- strates are fulfilled (glycosyl fluorides, azides,
tion of the activated donor substrate or or oxazolines). R : modification, for example,
1
further biocatalytic reaction cascades after C-6 oxidation.
transglycosylation. Glycosynthases can as
In transglycosylation, a kinetically preferred sugar substrate, being more reactive
than the product, is used as donor. Here p-nitrophenyl (pNP)-glycosides [46–48], or
disaccharides like lactose in high concentration [46] are suitable transglycosylation
substrates.
Endo- as well as exoglycosidases were successfully utilized in transglycosyla-
tion reactions adding di- or oligosaccharides (endoglycosidase) [49, 50] or single
carbohydrate units (exoglycosidase) [47] to a nucleophilic acceptor substrate like
alcohols, carbohydrates, or peptides. Both enzyme classes are readily available
from microbial sources or as recombinant enzymes. Even more beneficial, when
compared to GTs, is the inherent broader donor and acceptor substrate specificity
[51] giving rise to various neo-glycoconjugates [52, 53]. Based on their retaining
or inverting reaction mechanisms, glycosidases display absolute stereoselectivity
[44]; however, regioselectivity is not that strict and the formation of regioisomers
has been shown to be dependent on the source of the enzyme, the reaction time,
and the nature of the acceptor and donor used [44, 54]. Product hydrolysis is the
major reason for moderate product yields and the need for a tight reaction con-
trol over time using glycosidases [55]. During the reaction process, accumulating
products become substrates of the glycosidases and are subsequently hydrolyzed,