Page 173 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 173
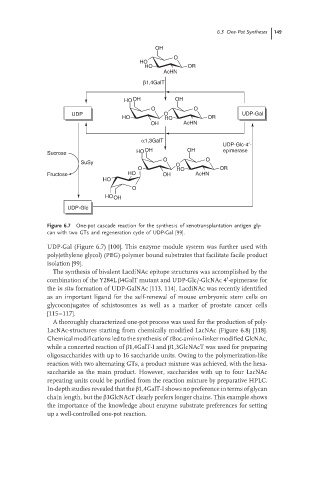
6.3 One-Pot Syntheses 149
OH
O
HO
HO OR
AcHN
β1,4GalT
HO OH OH
O O
UDP O UDP-Gal
HO HO OR
OH AcHN
α1,3GalT
UDP-Glc-4′-
HO OH OH epimerase
Sucrose
O O
SuSy O
O HO OR
Fructose HO OH AcHN
HO
O
HO OH
UDP-Glc
Figure 6.7 One-pot cascade reaction for the synthesis of xenotransplantation antigen gly-
can with two GTs and regeneration cycle of UDP-Gal [99].
UDP-Gal (Figure 6.7) [100]. This enzyme module system was further used with
poly(ethylene glycol) (PEG)-polymer bound substrates that facilitate facile product
isolation [99].
The synthesis of bivalent LacdiNAc epitope structures was accomplished by the
′
combination of the Y284L β4GalT mutant and UDP-Glc/-GlcNAc 4 -epimerase for
the in situ formation of UDP-GalNAc [113, 114]. LacdiNAc was recently identified
as an important ligand for the self-renewal of mouse embryonic stem cells on
glycoconjugates of schistosomes as well as a marker of prostate cancer cells
[115–117].
A thoroughly characterized one-pot process was used for the production of poly-
LacNAc-structures starting from chemically modified LacNAc (Figure 6.8) [118].
Chemical modifications led to the synthesis of tBoc-amino-linker modified GlcNAc,
while a concerted reaction of β1,4GalT-I and β1,3GlcNAcT was used for preparing
oligosaccharides with up to 16 saccharide units. Owing to the polymerization-like
reaction with two alternating GTs, a product mixture was achieved, with the hexa-
saccharide as the main product. However, saccharides with up to four LacNAc
repeating units could be purified from the reaction mixture by preparative HPLC.
In-depth studies revealed that the β1,4GalT-I shows no preference in terms of glycan
chain length, but the β3GlcNAcT clearly prefers longer chains. This example shows
the importance of the knowledge about enzyme substrate preferences for setting
up a well-controlled one-pot reaction.