Page 296 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 296
272 12 Mining Genomes for Nitrilases
I Environmental
isolates
Enzyme
partial Gene cloning
sequencing
Gene
amplification
Nitrilase Hypothetical
genes nitrilase
Gene sequences
synthesis
Enzyme Enzyme
overproduction characterization Database
mining
II Nitrilase Substrate
Experimentally Sequenced genomes
library screening confirmed
nitrilases
Nitrilase selection III
eDNA library
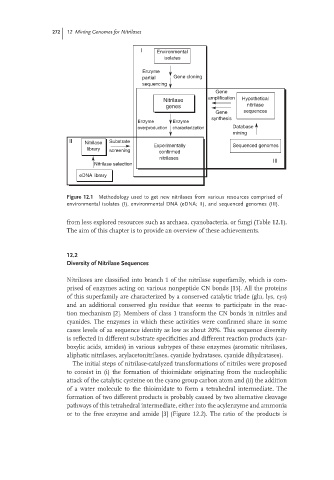
Figure 12.1 Methodology used to get new nitrilases from various resources comprised of
environmental isolates (I), environmental DNA (eDNA; II), and sequenced genomes (III).
from less explored resources such as archaea, cyanobacteria, or fungi (Table 12.1).
The aim of this chapter is to provide an overview of these achievements.
12.2
Diversity of Nitrilase Sequences
Nitrilases are classified into branch 1 of the nitrilase superfamily, which is com-
prised of enzymes acting on various nonpeptide CN bonds [15]. All the proteins
of this superfamily are characterized by a conserved catalytic triade (glu, lys, cys)
and an additional conserved glu residue that seems to participate in the reac-
tion mechanism [2]. Members of class 1 transform the CN bonds in nitriles and
cyanides. The enzymes in which these activities were confirmed share in some
cases levels of aa sequence identity as low as about 20%. This sequence diversity
is reflected in different substrate specificities and different reaction products (car-
boxylic acids, amides) in various subtypes of these enzymes (aromatic nitrilases,
aliphatic nitrilases, arylacetonitrilases, cyanide hydratases, cyanide dihydratases).
The initial steps of nitrilase-catalyzed transformations of nitriles were proposed
to consist in (i) the formation of thioimidate originating from the nucleophilic
attack of the catalytic cysteine on the cyano group carbon atom and (ii) the addition
of a water molecule to the thioimidate to form a tetrahedral intermediate. The
formation of two different products is probably caused by two alternative cleavage
pathways of this tetrahedral intermediate, either into the acylenzyme and ammonia
or to the free enzyme and amide [3] (Figure 12.2). The ratio of the products is