Page 261 - Biodegradable Polyesters
P. 261
10.1 Introduction 239
CH O CH O
3 3
O O
Direct HO O O poly
condensation
polymerization O CH 3 O CH
3
H Low molecular weight prepolymer Chain coupling agent
H 3 C M = 1000–5000
C OH w
C
HO
CH O CH 3 O
O 3
L-Lactic acid Azeotropic dehydration condensation O O
CH −H 2 O HO O OH
H 3
C OH O CH 3 O CH 3
C
HO Low molecular weight polymer
M > 100 000
w
O
D-Lactic acid
Polymerization
through CH O CH 3 O
lactide 3 O O CH 3
formation O O C C H
Ring-opening
HO O O poly polymerization
H C C
O CH O CH O O
3 3 H 3 C
Low molecular weight prepolymer
M w = 1 000–5 000 Lactide
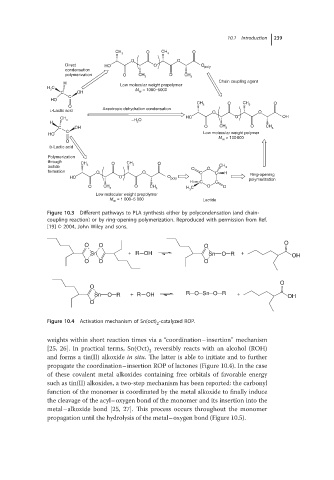
Figure 10.3 Different pathways to PLA synthesis either by polycondensation (and chain-
coupling reaction) or by ring-opening polymerization. Reproduced with permission from Ref.
[19] © 2004, John Wiley and sons.
O O O O
Sn + R OH Sn O R + OH
O O O
O
O
Sn O R + R OH R O Sn O R + OH
O
Figure 10.4 Activation mechanism of Sn(oct) -catalyzed ROP.
2
weights within short reaction times via a “coordination–insertion” mechanism
[25, 26]. In practical terms, Sn(Oct) reversibly reacts with an alcohol (ROH)
2
and forms a tin(II) alkoxide in situ. The latter is able to initiate and to further
propagate the coordination–insertion ROP of lactones (Figure 10.4). In the case
of these covalent metal alkoxides containing free orbitals of favorable energy
such as tin(II) alkoxides, a two-step mechanism has been reported: the carbonyl
function of the monomer is coordinated by the metal alkoxide to finally induce
the cleavage of the acyl–oxygen bond of the monomer and its insertion into the
metal–alkoxide bond [25, 27]. This process occurs throughout the monomer
propagation until the hydrolysis of the metal–oxygen bond (Figure 10.5).