Page 260 - Biodegradable Polyesters
P. 260
238 10 Highly Toughened Polylactide-Based Materials through Melt-Blending Techniques
O O
HO HO
OH OH
CH 3 CH 3
L-Lactic acid D-Lactic acid
O
O O
CH 3 CH 3 CH 3
O O O
O O H C O
H 3 C H 3 C 3
O O O
L,L-Lactide D,L-Lactide D,D-Lactide
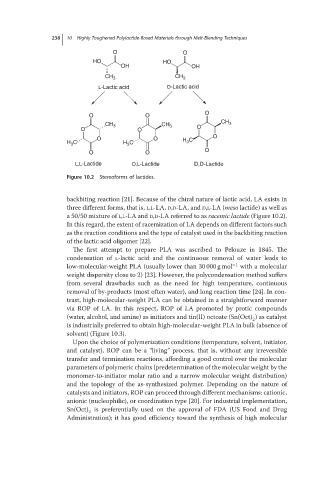
Figure 10.2 Stereoforms of lactides.
backbiting reaction [21]. Because of the chiral nature of lactic acid, LA exists in
three different forms, that is, L,L-LA, D,D-LA, and D,L-LA (meso lactide) as well as
a 50/50 mixture of L,L-LA and D,D-LA referred to as racemic lactide (Figure 10.2).
In this regard, the extent of racemization of LA depends on different factors such
as the reaction conditions and the type of catalyst used in the backbiting reaction
of the lactic acid oligomer [22].
The first attempt to prepare PLA was ascribed to Pelouze in 1845. The
condensation of L-lactic acid and the continuous removal of water leads to
low-molecular-weight PLA (usually lower than 30 000 g mol −1 with a molecular
weight dispersity close to 2) [23]. However, the polycondensation method suffers
from several drawbacks such as the need for high temperature, continuous
removal of by-products (most often water), and long reaction time [24]. In con-
trast, high-molecular-weight PLA can be obtained in a straightforward manner
via ROP of LA. In this respect, ROP of LA promoted by protic compounds
(water, alcohol, and amine) as initiators and tin(II) octoate (Sn(Oct) ) as catalyst
2
is industrially preferred to obtain high-molecular-weight PLA in bulk (absence of
solvent) (Figure 10.3).
Upon the choice of polymerization conditions (temperature, solvent, initiator,
and catalyst), ROP can be a “living” process, that is, without any irreversible
transfer and termination reactions, affording a good control over the molecular
parameters of polymeric chains (predetermination of the molecular weight by the
monomer-to-initiator molar ratio and a narrow molecular weight distribution)
and the topology of the as-synthesized polymer. Depending on the nature of
catalysts and initiators, ROP can proceed through different mechanisms: cationic,
anionic (nucleophilic), or coordination type [20]. For industrial implementation,
Sn(Oct) is preferentially used on the approval of FDA (US Food and Drug
2
Administration); it has good efficiency toward the synthesis of high molecular