Page 292 - Biofuels Refining and Performance
P. 292
Fuel Cells 271
life of 40,000 h with reasonable performance (degradation rate ∆V lifetime
(mV) 2 mV/1000 h) [3, 23].
9.3.5 Molten carbonate fuel cells (MCFCs)
The MCFC has evolved from work in the 1960s, aimed at producing a
fuel cell that would operate directly on coal [23, 24]. Although direct oper-
ation on coal is no longer a goal, a remarkable feature of the MCFC is
that it can directly operate on coal-derived fuel gases or natural gas and
is therefore also called a direct fuel cell (DFC). MCFCs operate at high
temperatures (600–650 C) compared to phosphoric acid (180–220 C) or
PEM fuel cells (60–85 C). Operation at high temperatures eliminates the
need for external fuel processors that the lower temperature fuel cells
require to extract hydrogen from naturally available fuel. When natu-
ral gas is used as fuel, methane (the main ingredient of natural gas) and
water (steam) are converted into a hydrogen-rich gas inside the MCFC
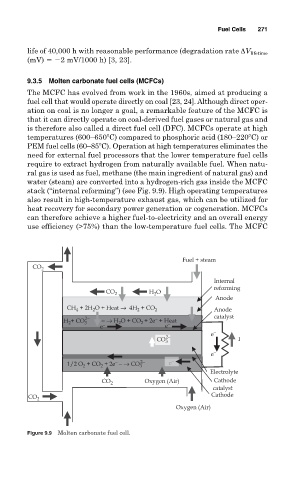
stack (“internal reforming”) (see Fig. 9.9). High operating temperatures
also result in high-temperature exhaust gas, which can be utilized for
heat recovery for secondary power generation or cogeneration. MCFCs
can therefore achieve a higher fuel-to-electricity and an overall energy
use efficiency (>75%) than the low-temperature fuel cells. The MCFC
CO 2
Internal
reforming
CO 2 H O
2
Anode
CH + 2H O + Heat → 4H + CO 2 Anode
2
2
4
2− − catalyst
H +CO 3 − → H O + CO + 2e + Heat
2
2
−
2
e e − e e
2− e −
CO 3 I
e −
−
1/2 O + CO + 2e − → CO 3 2− e −
2
2
Electrolyte
CO 2 Oxygen (Air) Cathode
catalyst
CO 2 Cathode
Oxygen (Air)
Figure 9.9 Molten carbonate fuel cell.