Page 289 - Biofuels Refining and Performance
P. 289
268 Chapter Nine
Electric current
e − e − Hydrogen molecule
Depleted e − − Oxygen molecule
hydrogen out e Water
Water
+
H 2 Hydrogen ion (H )
H O
2
H 2
O 2
Hydrogen in Oxygen in
Anode Cathode
Electrolyte
(Phosphoric acid) Cathode catalyst
Anode catalyst layer
layer
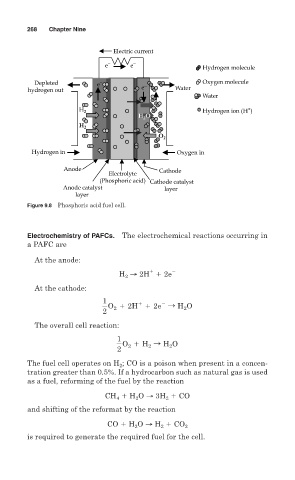
Figure 9.8 Phosphoric acid fuel cell.
Electrochemistry of PAFCs. The electrochemical reactions occurring in
a PAFC are
At the anode:
H → 2H 2e
2
At the cathode:
1
1 2
2
O 1 2H 1 2e S H 2 O
2
The overall cell reaction:
1
O 2 1 H 2 S H 2 O
2
The fuel cell operates on H ; CO is a poison when present in a concen-
2
tration greater than 0.5%. If a hydrocarbon such as natural gas is used
as a fuel, reforming of the fuel by the reaction
CH H O → 3H CO
2
2
4
and shifting of the reformat by the reaction
CO H O → H CO 2
2
2
is required to generate the required fuel for the cell.