Page 284 - Biofuels Refining and Performance
P. 284
Fuel Cells 263
design by improvement in the electrode structures, better electrocata-
lysts, more conductive electrolytes, thinner cell components, and so forth.
It is possible to improve the cell performance by modifying the operat-
ing conditions such as higher gas pressure, higher temperature, and a
change in gas composition to lower the gas impurity concentration [3].
9.3.2 Direct methanol fuel cells (DMFCs)
Direct methanol fuel cells are similar to the PEMFC as they also use a
polymer membrane as the electrolyte. However, it produces power by
direct conversion of liquid methanol to hydrogen ions on the anode side
of the fuel cell. In the DMFC, the anode catalyst draws hydrogen directly
from the liquid methanol, thus eliminating the need for a fuel reformer.
All the DMFC components (anode, cathode, membrane, and catalysts)
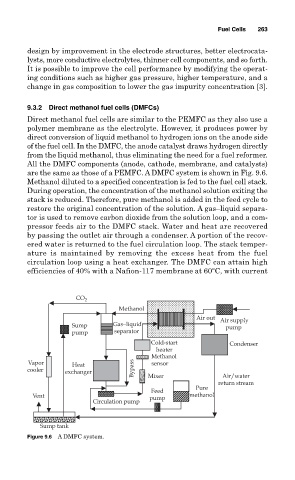
are the same as those of a PEMFC. A DMFC system is shown in Fig. 9.6.
Methanol diluted to a specified concentration is fed to the fuel cell stack.
During operation, the concentration of the methanol solution exiting the
stack is reduced. Therefore, pure methanol is added in the feed cycle to
restore the original concentration of the solution. A gas–liquid separa-
tor is used to remove carbon dioxide from the solution loop, and a com-
pressor feeds air to the DMFC stack. Water and heat are recovered
by passing the outlet air through a condenser. A portion of the recov-
ered water is returned to the fuel circulation loop. The stack temper-
ature is maintained by removing the excess heat from the fuel
circulation loop using a heat exchanger. The DMFC can attain high
efficiencies of 40% with a Nafion-117 membrane at 60 C, with current
CO 2
Methanol
Air out Air supply
Sump Gas–liquid pump
pump separator
Cold-start Condenser
heater
Methanol
Vapor Heat sensor
cooler exchanger Bypass Mixer Air/water
return stream
Pure
Feed
Vent pump methanol
Circulation pump
Sump tank
Figure 9.6 A DMFC system.