Page 282 - Biofuels Refining and Performance
P. 282
Fuel Cells 261
The polarization losses result in a further decrease in actual cell volt-
age (V) from its ideal potential E (V E potential drop due to losses).
The activation polarization loss is dominant at low current density. This
is because electronic barriers have to be overcome prior to current and
ion flows. Activation polarization is present when the rate of an electro-
chemical reaction at an electrode surface is controlled by sluggish elec-
trode kinetics. Therefore, activation polarization is directly related to the
rates of electrochemical reactions. In an electrochemical reaction with
> 50 100 mV, activation polarization is described by a semi-
h act
empirical equation known as the Tafel equation:
RT i
h act 5 a b ln a b
nF i 0
where is the electron transfer coefficient of the reaction at the elec-
trode (anode or cathode), and i is the exchange current density. The Tafel
0
slope for the PEMFC electrochemical reaction is about 100 mV/decade
at room temperature. Thus there is an incentive to develop electrocat-
alysts that yield a lower Tafel slope [2, 3, 11, 12].
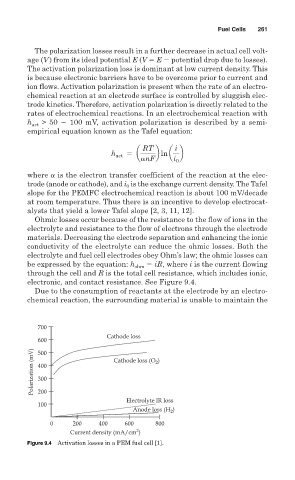
Ohmic losses occur because of the resistance to the flow of ions in the
electrolyte and resistance to the flow of electrons through the electrode
materials. Decreasing the electrode separation and enhancing the ionic
conductivity of the electrolyte can reduce the ohmic losses. Both the
electrolyte and fuel cell electrodes obey Ohm’s law; the ohmic losses can
be expressed by the equation: h ohm iR, where i is the current flowing
through the cell and R is the total cell resistance, which includes ionic,
electronic, and contact resistance. See Figure 9.4.
Due to the consumption of reactants at the electrode by an electro-
chemical reaction, the surrounding material is unable to maintain the
700
Cathode loss
600
Polarization (mV) 400 Cathode loss (O )
500
2
300
200
Electrolyte IR loss
100
Anode loss (H )
2
0 200 400 600 800
2
Current density (mA/cm )
Figure 9.4 Activation losses in a PEM fuel cell [1].