Page 286 - Biofuels Refining and Performance
P. 286
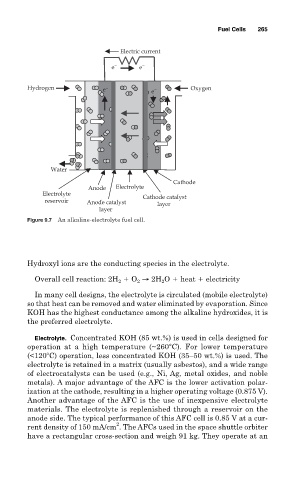
Fuel Cells 265
Electric current
e − e −
Hydrogen e − e − Oxygen
Water
Cathode
Anode Electrolyte
Electrolyte Cathode catalyst
reservoir Anode catalyst layer
layer
Figure 9.7 An alkaline-electrolyte fuel cell.
Hydroxyl ions are the conducting species in the electrolyte.
Overall cell reaction: 2H O → 2H O heat electricity
2
2
2
In many cell designs, the electrolyte is circulated (mobile electrolyte)
so that heat can be removed and water eliminated by evaporation. Since
KOH has the highest conductance among the alkaline hydroxides, it is
the preferred electrolyte.
Electrolyte. Concentrated KOH (85 wt.%) is used in cells designed for
operation at a high temperature (~260 C). For lower temperature
(<120 C) operation, less concentrated KOH (35–50 wt.%) is used. The
electrolyte is retained in a matrix (usually asbestos), and a wide range
of electrocatalysts can be used (e.g., Ni, Ag, metal oxides, and noble
metals). A major advantage of the AFC is the lower activation polar-
ization at the cathode, resulting in a higher operating voltage (0.875 V).
Another advantage of the AFC is the use of inexpensive electrolyte
materials. The electrolyte is replenished through a reservoir on the
anode side. The typical performance of this AFC cell is 0.85 V at a cur-
2
rent density of 150 mA/cm . The AFCs used in the space shuttle orbiter
have a rectangular cross-section and weigh 91 kg. They operate at an