Page 303 - Biofuels Refining and Performance
P. 303
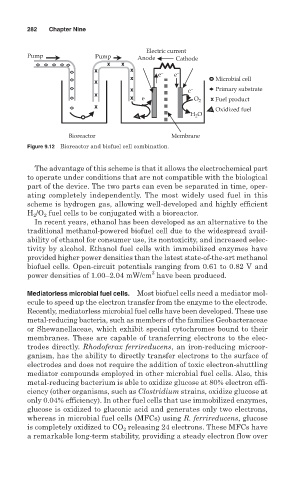
282 Chapter Nine
Electric current
Pump Pump Anode Cathode
e − e −
Microbial cell
− Primary substrate
e
e − O 2 Fuel product
Oxidized fuel
H O
2
Bioreactor Membrane
Figure 9.12 Bioreactor and biofuel cell combination.
The advantage of this scheme is that it allows the electrochemical part
to operate under conditions that are not compatible with the biological
part of the device. The two parts can even be separated in time, oper-
ating completely independently. The most widely used fuel in this
scheme is hydrogen gas, allowing well-developed and highly efficient
/O fuel cells to be conjugated with a bioreactor.
H 2 2
In recent years, ethanol has been developed as an alternative to the
traditional methanol-powered biofuel cell due to the widespread avail-
ability of ethanol for consumer use, its nontoxicity, and increased selec-
tivity by alcohol. Ethanol fuel cells with immobilized enzymes have
provided higher power densities than the latest state-of-the-art methanol
biofuel cells. Open-circuit potentials ranging from 0.61 to 0.82 V and
2
power densities of 1.00–2.04 mW/cm have been produced.
Mediatorless microbial fuel cells. Most biofuel cells need a mediator mol-
ecule to speed up the electron transfer from the enzyme to the electrode.
Recently, mediatorless microbial fuel cells have been developed. These use
metal-reducing bacteria, such as members of the families Geobacteraceae
or Shewanellaceae, which exhibit special cytochromes bound to their
membranes. These are capable of transferring electrons to the elec-
trodes directly. Rhodoferax ferrireducens, an iron-reducing microor-
ganism, has the ability to directly transfer electrons to the surface of
electrodes and does not require the addition of toxic electron-shuttling
mediator compounds employed in other microbial fuel cells. Also, this
metal-reducing bacterium is able to oxidize glucose at 80% electron effi-
ciency (other organisms, such as Clostridium strains, oxidize glucose at
only 0.04% efficiency). In other fuel cells that use immobilized enzymes,
glucose is oxidized to gluconic acid and generates only two electrons,
whereas in microbial fuel cells (MFCs) using R. ferrireducens, glucose
is completely oxidized to CO releasing 24 electrons. These MFCs have
2
a remarkable long-term stability, providing a steady electron flow over