Page 301 - Biofuels Refining and Performance
P. 301
280 Chapter Nine
is the electrical coupling of the biological components of the system with
the fuel cell electrodes. Molecules known as electron-transfer mediators
are needed to provide efficient transport of electrons between the bio-
logical components (enzymes or microbial cells) and the electrodes of the
biofuel cell. Integrated biocatalytic systems that include biocatalysts,
electron-transfer mediators, and electrodes are under research and
development. Biofuel cells have much wider fuel options; enzymatic bio-
fuel cells can operate on a wide variety of available fuels such as ethanol,
sugars, or even waste materials.
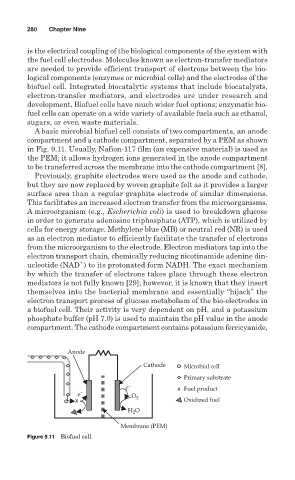
A basic microbial biofuel cell consists of two compartments, an anode
compartment and a cathode compartment, separated by a PEM as shown
in Fig. 9.11. Usually, Nafion-117 film (an expensive material) is used as
the PEM; it allows hydrogen ions generated in the anode compartment
to be transferred across the membrane into the cathode compartment [8].
Previously, graphite electrodes were used as the anode and cathode,
but they are now replaced by woven graphite felt as it provides a larger
surface area than a regular graphite electrode of similar dimensions.
This facilitates an increased electron transfer from the microorganisms.
A microorganism (e.g., Escherichia coli) is used to breakdown glucose
in order to generate adenosine triphosphate (ATP), which is utilized by
cells for energy storage. Methylene blue (MB) or neutral red (NR) is used
as an electron mediator to efficiently facilitate the transfer of electrons
from the microorganism to the electrode. Electron mediators tap into the
electron transport chain, chemically reducing nicotinamide adenine din-
ucleotide (NAD ) to its protonated form NADH. The exact mechanism
by which the transfer of electrons takes place through these electron
mediators is not fully known [29]; however, it is known that they insert
themselves into the bacterial membrane and essentially “hijack” the
electron transport process of glucose metabolism of the bio-electrodes in
a biofuel cell. Their activity is very dependent on pH, and a potassium
phosphate buffer (pH 7.0) is used to maintain the pH value in the anode
compartment. The cathode compartment contains potassium ferricyanide,
Anode
Cathode Microbial cell
Primary substrate
e − x Fuel product
e − O
x 2 Oxidized fuel
H O
2
Membrane (PEM)
Figure 9.11 Biofuel cell.