Page 176 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 176
Chapter | 5 Pyrolysis 153
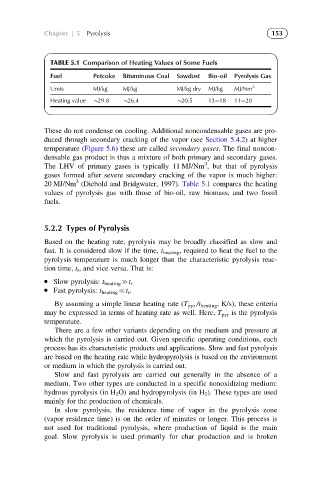
TABLE 5.1 Comparison of Heating Values of Some Fuels
Fuel Petcoke Bituminous Coal Sawdust Bio-oil Pyrolysis Gas
Units MJ/kg MJ/kg MJ/kg dry MJ/kg MJ/Nm 3
Heating value B29.8 B26.4 B20.5 13 18 11 20
These do not condense on cooling. Additional noncondensable gases are pro-
duced through secondary cracking of the vapor (see Section 5.4.2) at higher
temperature (Figure 5.6) these are called secondary gases. The final noncon-
densable gas product is thus a mixture of both primary and secondary gases.
3
The LHV of primary gases is typically 11 MJ/Nm , but that of pyrolysis
gases formed after severe secondary cracking of the vapor is much higher:
3
20 MJ/Nm (Diebold and Bridgwater, 1997). Table 5.1 compares the heating
values of pyrolysis gas with those of bio-oil, raw biomass, and two fossil
fuels.
5.2.2 Types of Pyrolysis
Based on the heating rate, pyrolysis may be broadly classified as slow and
fast. It is considered slow if the time, t heating , required to heat the fuel to the
pyrolysis temperature is much longer than the characteristic pyrolysis reac-
tion time, t r , and vice versa. That is:
Slow pyrolysis: t heating ct r
Fast pyrolysis: t heating {t r .
By assuming a simple linear heating rate (T pyr /t heating , K/s), these criteria
may be expressed in terms of heating rate as well. Here, T pyr is the pyrolysis
temperature.
There are a few other variants depending on the medium and pressure at
which the pyrolysis is carried out. Given specific operating conditions, each
process has its characteristic products and applications. Slow and fast pyrolysis
are based on the heating rate while hydropyrolysis is based on the environment
or medium in which the pyrolysis is carried out.
Slow and fast pyrolysis are carried out generally in the absence of a
medium. Two other types are conducted in a specific nonoxidizing medium:
hydrous pyrolysis (in H 2 O) and hydropyrolysis (in H 2 ). These types are used
mainly for the production of chemicals.
In slow pyrolysis, the residence time of vapor in the pyrolysis zone
(vapor residence time) is on the order of minutes or longer. This process is
not used for traditional pyrolysis, where production of liquid is the main
goal. Slow pyrolysis is used primarily for char production and is broken