Page 359 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 359
336 BIOMATERIALS
studied in the literature. Table 13.7 shows physical properties for PHAs compared to polylactide,
polyglycolide, and their copolymers.
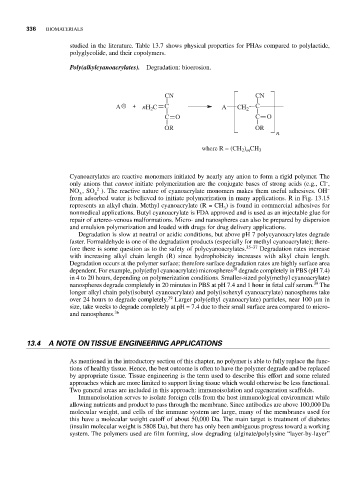
Poly(alkylcyanoacrylates). Degradation: bioerosion.
CN CN
A nH C C A CH 2 C
2
CO C O
OR OR
n
where R = (CH ) CH 3
2 m
Cyanoacrylates are reactive monomers initiated by nearly any anion to form a rigid polymer. The
–
only anions that cannot initiate polymerization are the conjugate bases of strong acids (e.g., Cl ,
–
NO , SO 2– ). The reactive nature of cyanoacrylate monomers makes them useful adhesives. OH −
3 4
from adsorbed water is believed to initiate polymerization in many applications. R in Fig. 13.15
represents an alkyl chain. Methyl cyanoacrylate (R = CH ) is found in commercial adhesives for
3
nonmedical applications. Butyl cyanoacrylate is FDA approved and is used as an injectable glue for
repair of artereo-venous malformations. Micro- and nanospheres can also be prepared by dispersion
and emulsion polymerization and loaded with drugs for drug delivery applications.
Degradation is slow at neutral or acidic conditions, but above pH 7 polycyanoacrylates degrade
faster. Formaldehyde is one of the degradation products (especially for methyl cyanoacrylate); there-
fore there is some question as to the safety of polycyanoacrylates. 35–37 Degradation rates increase
with increasing alkyl chain length (R) since hydrophobicity increases with alkyl chain length.
Degradation occurs at the polymer surface; therefore surface degradation rates are highly surface area
38
dependent. For example, poly(ethyl cyanoacrylate) microspheres degrade completely in PBS (pH 7.4)
in 4 to 20 hours, depending on polymerization conditions. Smaller-sized poly(methyl cyanoacrylate)
39
nanospheres degrade completely in 20 minutes in PBS at pH 7.4 and 1 hour in fetal calf serum. The
longer alkyl chain poly(isobutyl cyanoacrylate) and poly(isohexyl cyanoacrylate) nanospheres take
over 24 hours to degrade completely. 39 Larger poly(ethyl cyanoacrylate) particles, near 100 μm in
size, take weeks to degrade completely at pH = 7.4 due to their small surface area compared to micro-
and nanospheres. 36
13.4 A NOTE ON TISSUE ENGINEERING APPLICATIONS
As mentioned in the introductory section of this chapter, no polymer is able to fully replace the func-
tions of healthy tissue. Hence, the best outcome is often to have the polymer degrade and be replaced
by appropriate tissue. Tissue engineering is the term used to describe this effort and some related
approaches which are more limited to support living tissue which would otherwise be less functional.
Two general areas are included in this approach: immunoisolation and regeneration scaffolds.
Immunoisolation serves to isolate foreign cells from the host immunological environment while
allowing nutrients and product to pass through the membrane. Since antibodies are above 100,000 Da
molecular weight, and cells of the immune system are large, many of the membranes used for
this have a molecular weight cutoff of about 50,000 Da. The main target is treatment of diabetes
(insulin molecular weight is 5808 Da), but there has only been ambiguous progress toward a working
system. The polymers used are film forming, slow degrading (alginate/polylysine “layer-by-layer”