Page 136 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 136
DESIGN OF RESPIRATORY DEVICES 115
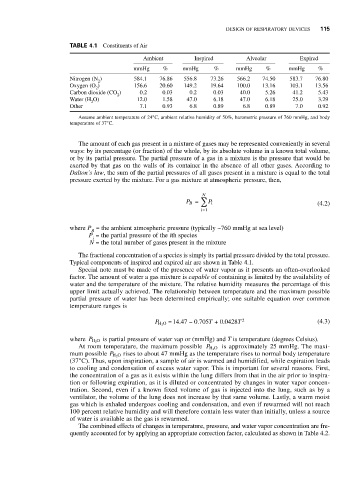
TABLE 4.1 Constituents of Air
Ambient Inspired Alveolar Expired
mmHg % mmHg % mmHg % mmHg %
Nitrogen (N ) 584.1 76.86 556.8 73.26 566.2 74.50 583.7 76.80
2
Oxygen (O ) 156.6 20.60 149.2 19.64 100.0 13.16 103.1 13.56
2
Carbon dioxide (CO ) 0.2 0.03 0.2 0.03 40.0 5.26 41.2 5.43
2
Water (H O) 12.0 1.58 47.0 6.18 47.0 6.18 25.0 3.29
2
Other 7.1 0.93 6.8 0.89 6.8 0.89 7.0 0.92
Assume ambient temperature of 24°C, ambient relative humidity of 50%, barometric pressure of 760 mmHg, and body
temperature of 37°C.
The amount of each gas present in a mixture of gases may be represented conveniently in several
ways: by its percentage (or fraction) of the whole, by its absolute volume in a known total volume,
or by its partial pressure. The partial pressure of a gas in a mixture is the pressure that would be
exerted by that gas on the walls of its container in the absence of all other gases. According to
Dalton’s law, the sum of the partial pressures of all gases present in a mixture is equal to the total
pressure exerted by the mixture. For a gas mixture at atmospheric pressure, then,
N
P B = ∑ P i (4.2)
i=1
where P = the ambient atmospheric pressure (typically ~760 mmHg at sea level)
B
P = the partial pressure of the ith species
i
N = the total number of gases present in the mixture
The fractional concentration of a species is simply its partial pressure divided by the total pressure.
Typical components of inspired and expired air are shown in Table 4.1.
Special note must be made of the presence of water vapor as it presents an often-overlooked
factor. The amount of water a gas mixture is capable of containing is limited by the availability of
water and the temperature of the mixture. The relative humidity measures the percentage of this
upper limit actually achieved. The relationship between temperature and the maximum possible
partial pressure of water has been determined empirically; one suitable equation over common
temperature ranges is
=
P HO 14 47 − 0 705 T + 0 0428 T 2 (4.3)
.
.
.
2
where P HO is partial pressure of water vap or (mmHg) and T is temperature (degrees Celsius).
2
At room temperature, the maximum possible P HO is approximately 25 mmHg. The maxi-
2
mum possible P HO rises to about 47 mmHg as the temperature rises to normal body temperature
2
(37°C). Thus, upon inspiration, a sample of air is warmed and humidified, while expiration leads
to cooling and condensation of excess water vapor. This is important for several reasons. First,
the concentration of a gas as it exists within the lung differs from that in the air prior to inspira-
tion or following expiration, as it is diluted or concentrated by changes in water vapor concen-
tration. Second, even if a known fixed volume of gas is injected into the lung, such as by a
ventilator, the volume of the lung does not increase by that same volume. Lastly, a warm moist
gas which is exhaled undergoes cooling and condensation, and even if rewarmed will not reach
100 percent relative humidity and will therefore contain less water than initially, unless a source
of water is available as the gas is rewarmed.
The combined effects of changes in temperature, pressure, and water vapor concentration are fre-
quently accounted for by applying an appropriate correction factor, calculated as shown in Table 4.2.