Page 165 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 165
144 MEDICAL DEVICE DESIGN
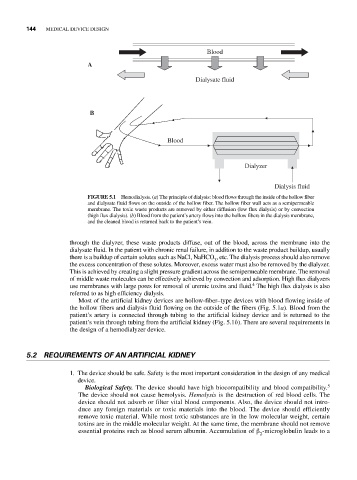
Blood
A
Dialysate fluid
B
Blood
Dialyzer
Dialysis fluid
FIGURE 5.1 Hemodialysis. (a) The principle of dialysis: blood flows through the inside of the hollow fiber
and dialysate fluid flows on the outside of the hollow fiber. The hollow fiber wall acts as a semipermeable
membrane. The toxic waste products are removed by either diffusion (low flux dialysis) or by convection
(high flux dialysis). (b) Blood from the patient’s artery flows into the hollow fibers in the dialysis membrane,
and the cleaned blood is returned back to the patient’s vein.
through the dialyzer, these waste products diffuse, out of the blood, across the membrane into the
dialysate fluid. In the patient with chronic renal failure, in addition to the waste product buildup, usually
there is a buildup of certain solutes such as NaCl, NaHCO , etc. The dialysis process should also remove
3
the excess concentration of these solutes. Moreover, excess water must also be removed by the dialyzer.
This is achieved by creating a slight pressure gradient across the semipermeable membrane. The removal
of middle waste molecules can be effectively achieved by convection and adsorption. High flux dialyzers
4
use membranes with large pores for removal of uremic toxins and fluid. The high flux dialysis is also
referred to as high efficiency dialysis.
Most of the artificial kidney devices are hollow-fiber–type devices with blood flowing inside of
the hollow fibers and dialysis fluid flowing on the outside of the fibers (Fig. 5.1a). Blood from the
patient’s artery is connected through tubing to the artificial kidney device and is returned to the
patient’s vein through tubing from the artificial kidney (Fig. 5.1b). There are several requirements in
the design of a hemodialyzer device.
5.2 REQUIREMENTS OF AN ARTIFICIAL KIDNEY
1. The device should be safe. Safety is the most important consideration in the design of any medical
device.
Biological Safety. The device should have high biocompatibility and blood compatibility. 5
The device should not cause hemolysis. Hemolysis is the destruction of red blood cells. The
device should not adsorb or filter vital blood components. Also, the device should not intro-
duce any foreign materials or toxic materials into the blood. The device should efficiently
remove toxic material. While most toxic substances are in the low molecular weight, certain
toxins are in the middle molecular weight. At the same time, the membrane should not remove
essential proteins such as blood serum albumin. Accumulation of β -microglobulin leads to a
2