Page 183 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 183
162 MEDICAL DEVICE DESIGN
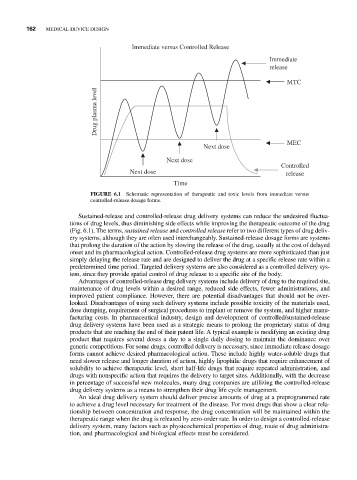
Immediate versus Controlled Release
Immediate
release
MTC
Drug plasma level
MEC
Next dose
Next dose
Controlled
Next dose release
Time
FIGURE 6.1 Schematic representation of therapeutic and toxic levels from immediate versus
controlled-release dosage forms.
Sustained-release and controlled-release drug delivery systems can reduce the undesired fluctua-
tions of drug levels, thus diminishing side effects while improving the therapeutic outcome of the drug
(Fig. 6.1). The terms, sustained release and controlled release refer to two different types of drug deliv-
ery systems, although they are often used interchangeably. Sustained-release dosage forms are systems
that prolong the duration of the action by slowing the release of the drug, usually at the cost of delayed
onset and its pharmacological action. Controlled-release drug systems are more sophisticated than just
simply delaying the release rate and are designed to deliver the drug at a specific release rate within a
predetermined time period. Targeted delivery systems are also considered as a controlled delivery sys-
tem, since they provide spatial control of drug release to a specific site of the body.
Advantages of controlled-release drug delivery systems include delivery of drug to the required site,
maintenance of drug levels within a desired range, reduced side effects, fewer administrations, and
improved patient compliance. However, there are potential disadvantages that should not be over-
looked. Disadvantages of using such delivery systems include possible toxicity of the materials used,
dose dumping, requirement of surgical procedures to implant or remove the system, and higher manu-
facturing costs. In pharmaceutical industry, design and development of controlled/sustained-release
drug delivery systems have been used as a strategic means to prolong the proprietary status of drug
products that are reaching the end of their patent life. A typical example is modifying an existing drug
product that requires several doses a day to a single daily dosing to maintain the dominance over
generic competitions. For some drugs, controlled delivery is necessary, since immediate release dosage
forms cannot achieve desired pharmacological action. These include highly water-soluble drugs that
need slower release and longer duration of action, highly lipophilic drugs that require enhancement of
solubility to achieve therapeutic level, short half-life drugs that require repeated administration, and
drugs with nonspecific action that requires the delivery to target sites. Additionally, with the decrease
in percentage of successful new molecules, many drug companies are utilizing the controlled-release
drug delivery systems as a means to strengthen their drug life cycle management.
An ideal drug delivery system should deliver precise amounts of drug at a preprogrammed rate
to achieve a drug level necessary for treatment of the disease. For most drugs that show a clear rela-
tionship between concentration and response, the drug concentration will be maintained within the
therapeutic range when the drug is released by zero-order rate. In order to design a controlled-release
delivery system, many factors such as physicochemical properties of drug, route of drug administra-
tion, and pharmacological and biological effects must be considered.