Page 86 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 86
OVERVIEW OF CARDIOVASCULAR DEVICES 65
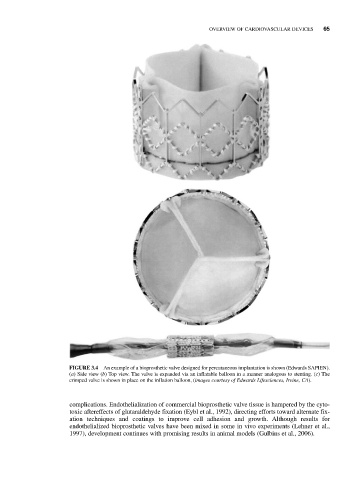
FIGURE 3.4 An example of a bioprosthetic valve designed for percutaneous implantation is shown (Edwards SAPIEN).
(a) Side view (b) Top view. The valve is expanded via an inflatable balloon in a manner analogous to stenting. (c) The
crimped valve is shown in place on the inflation balloon, (images courtesy of Edwards Lifesciences, Irvine, CA).
complications. Endothelialization of commercial bioprosthetic valve tissue is hampered by the cyto-
toxic aftereffects of glutaraldehyde fixation (Eybl et al., 1992), directing efforts toward alternate fix-
ation techniques and coatings to improve cell adhesion and growth. Although results for
endothelialized bioprosthetic valves have been mixed in some in vivo experiments (Lehner et al.,
1997), development continues with promising results in animal models (Gulbins et al., 2006).