Page 33 - Caldera Volcanism Analysis, Modelling and Response
P. 33
8 Fidel Costa
the onset of cooling. Later, Dodson (1986) introduced the closure profile which
allows for different zones of a crystal to ‘close’ at different temperatures (e.g.,
centres at higher temperature than rims). Ganguly and Tirone (1999, 2001)
presented new formulations in which the closure profiles and temperatures can be
calculated for minerals with an arbitrary extent of diffusion. This effectively
eliminates condition (4) (above) and thus should be of wide applicability to igneous
systems. Using the Dodson (1973, 1986) equations when condition (4) does not
apply leads to higher closure temperatures than the correct values (see Ganguly and
Tirone, 1999, 2001).
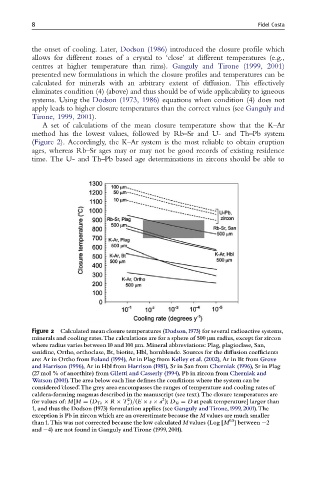
A set of calculations of the mean closure temperature show that the K–Ar
method has the lowest values, followed by Rb–Sr and U- and Th–Pb system
(Figure 2). Accordingly, the K–Ar system is the most reliable to obtain eruption
ages, whereas Rb–Sr ages may or may not be good records of existing residence
time. The U- and Th–Pb based age determinations in zircons should be able to
Figure 2 Calculated mean closure temperatures (Dodson,1973) for several radioactive systems,
minerals and cooling rates.The calculations are for a sphere of 500 mm radius, except for zircon
where radius varies between 10 and 100 mm. Mineral abbreviations: Plag, plagioclase, San,
sanidine, Ortho, orthoclase, Bt, biotite, Hbl, hornblende. Sources for the di¡usion coe⁄cients
are: Ar in Ortho from Foland (1994),Ar in Plagfrom Kelley et al. (2002),Ar in Btfrom Grove
and Harrison (1996), Ar in Hbl from Harrison (1981),Sr inSanfrom Cherniak (1996),Sr inPlag
(27 mol % of anorthite) from Giletti and Casserly (1994), Pb inzirconfrom Cherniak and
Watson (2001).The area below each line de¢nes the conditions where the system can be
considered‘closed’.The grey area encompasses the ranges of temperature and cooling rates of
caldera-forming magmas described in the manuscript (see text).The closure temperatures are
2
2
for values of: M½M ¼ðD To R T Þ=ðE s a Þ; D To ¼ D at peak temperature] larger than
c
1, and thus the Dodson (1973) formulation applies (see Ganguly andTirone,1999, 2001).The
exception is Pb in zircon which are an overestimate because the M values are much smaller
0.5
than 1.This was not corrected because the low calculated M values (Log [M ] between 2
and 4) are not found in Ganguly andTirone (1999, 2001).