Page 148 - Carbon Nanotubes
P. 148
ter only at the open ends[42]. The inner tubules ap- nickel and iron[21]) is introduced into the arc synthe-
pear to be protected by the outer layers and survive sis as a mixture of graphite and pure metal powders
the purification process. pressed into a hole bored in the center of the graph-
Similar results were found by Bacsa et a/. [26] for ite anode. The cathode is translated to maintain a
cathode core material. Raman scattering spectra were fixed gap and stable current as the anode is vaporized
reported by these authors for material shown in these in a helium atmosphere. In the case of nickel and iron,
figures, and these results are discussed below. Their methane is added to the otherwise inert helium atmo-
HRTEM images showed that heating core material in sphere. Nanotubes are found in carbonaceous mate-
air induces a clear reduction in the relative abundance rial condensed on the water-cooled walls and also in
of the carbon nanoparticles. The Raman spectrum of cobweb-like structures that form throughout the arc
these nanoparticles would be expected to resemble an chamber. Bright-field TEM images (100,000~) of the
intermediate between a strongly disordered carbon Co-catalyzed, arc-derived carbon material reveal nu-
black synthesized at -850°C (Fig. 2d) and that of car- merous narrow-diameter single-wall nanotubes and
bon black graphitized in an inert atmosphere at 2820°C small Co particles with diameters in the range 10-50 nm
(Fig. 2c). As discussed above in section 2, the small surrounded by a thick (-50 nm) carbon coating[U].
particle size, as well as structural disorder in the small
particles (dia. -200 A), activates the D-band Raman 4.2 Raman scattering from nested
scattering near 1350 cm-' . carbon nanotubes
Small diameter, single-wall nanotubes have been Several Raman studies have been carried out on
synthesized with metal catalysts by maintaining a dc nested nanotubes[23-261. The first report was by Hiura
arc (30 V, 95 A) between two electrodes in -300 Torr et al. [23], who observed a strong first-order band at
of He gas.[21,22] The metal catalyst (cobalt[22] or 1574 cm-I and a weaker, broader D-band at 1346
I I I I
40- I I I 1 I - 40tll I 1 1 1 1 IIIII -
I I I 1 I 111 I I I I I
I 01 1 Ld
0: I 400 800 1200 1600 0 400 800 1200 1600
Frequency (cm-1) Frequency (cm-1)
(a) (b)
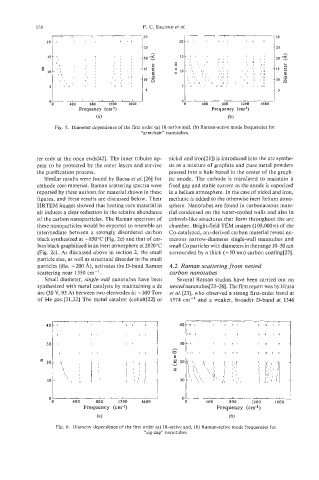
Fig. 6. Diameter dependence of the first order (a) IR-active and, (b) Raman-active mode frequencies for
"zig-zag" nanotubes.