Page 157 - Carbonate Sedimentology and Sequence Stratigraphy
P. 157
148 WOLFGANG SCHLAGER
the lack of protecting rims, leads to wholesale reworking of to be preserved (e.g. Nelson and James, 2000, p. 616).
shelf sediment when sea level falls and the high-energy belt
shifts downward.
Reworking notwithstanding, the internal anatomy of
cool-water carbonate deposits often records shoaling or
deepening trends that aid in recognizing systems tracts. B O
Shoaling cycles bounded by exposure or flooding surfaces O B
are particularly common (Fig. 8.1; James, 1997; Knoerich and
Mutti, 2003). Changes in water depth can be gleaned from
organisms (e.g. Betzler 1997; James et al. 2001; Brachert et below above below discontin- within (near) in within stacked
unconformities uities capping situ biomounds (cross-bedded)
al., 2003), and from hydrodynamic structures. Such struc- subtidal cycles sand bodies
tures are more common than in tropical carbonates because marine cemented horizons
the sediment consists mainly of sand and gravel and is less
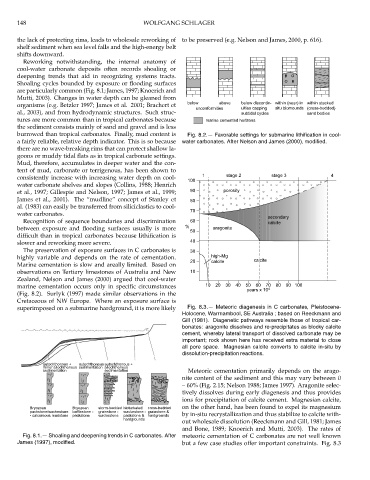
burrowed than tropical carbonates. Finally, mud content is Fig. 8.2.— Favorable settings for submarine lithification in cool-
a fairly reliable, relative depth indicator. This is so because water carbonates. After Nelson and James (2000), modified.
there are no wave-breaking rims that can protect shallow la-
goons or muddy tidal flats as in tropical carbonate settings.
Mud, therefore, accumulates in deeper water and the con-
tent of mud, carbonate or terrigenous, has been shown to
consistently increase with increasing water depth on cool- 1 stage 2 stage 3 4
100
water carbonate shelves and slopes (Collins, 1988; Henrich
et al., 1997; Gillespie and Nelson, 1997; James et al., 1999; 90 porosity
James et al., 2001). The “mudline” concept of Stanley et 80
al. (1983) can easily be transferred from siliciclastics to cool-
70
water carbonates.
secondary
Recognition of sequence boundaries and discrimination 60 calcite
between exposure and flooding surfaces usually is more % aragonite
50
difficult than in tropical carbonates because lithification is
slower and reworking more severe. 40
The preservation of exposure surfaces in C carbonates is 30
highly variable and depends on the rate of cementation. high-Mg
20 calcite calcite
Marine cementation is slow and areally limited. Based on
observations on Tertiary limestones of Australia and New 10
Zealand, Nelson and James (2000) argued that cool-water
marine cementation occurs only in specific circumstances 10 20 30 40 50 60 70 80 90 100
years x 10
4
(Fig. 8.2). Surlyk (1997) made similar observations in the
Cretaceous of NW Europe. Where an exposure surface is
superimposed on a submarine hardground, it is more likely Fig. 8.3.— Meteoric diagenesis in C carbonates, Pleistocene-
Holocene, Warrnambool, SE Australia ; based on Reeckmann and
Gill (1981). Diagenetic pathways resemble those of tropical car-
bonates: aragonite dissolves and re-precipitates as blocky calcite
cement, whereby lateral transport of dissolved carbonate may be
important; rock shown here has received extra material to close
all pore space. Magnesian calcite converts to calcite in-situ by
dissolution-precipitation reactions.
autochthonous + autochthonous autochthonous +
minor allochthonous sedimentation allochthonous
sedimentation sedimentation Meteoric cementation primarily depends on the arago-
Y
Y Y Y
nite content of the sediment and this may vary between 0
Y Y
Y Y Y Y Y Y – 60% (Fig. 2.15; Nelson 1988; James 1997). Aragonite selec-
tively dissolves during early diagenesis and thus provides
Y Y Y
Y Y Y
ions for precipitation of calcite cement. Magnesian calcite,
Bryozoan Bryozoan storm-bedded bioturbated cross-bedded on the other hand, has been found to expel its magnesium
packstone/wackestone bafflestone - grainstone - wackestone - grainstone &
- calcareous mudstone packstone wackestone packstone & hardgrounds by in-situ recrystallization and thus stabilize to calcite with-
hardgrounds
out wholesale dissolution (Reeckmann and Gill, 1981; James
and Bone, 1989; Knoerich and Mutti, 2003). The rates of
Fig. 8.1.— Shoaling and deepening trends in C carbonates. After meteoric cementation of C carbonates are not well known
James (1997), modified. but a few case studies offer important constraints. Fig. 8.3