Page 131 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 131
94 Carraher’s Polymer Chemistry
R
R 1
R R R 1 R R 1 R
R R R R R
R 1 R R 1 R R
R R 1 R R 1
R 1 R 1 R R 1
R R R R 1
R 1
R
R 1 R 1 R
R 1
R R 1 R 1 R 1
R
R R 1 R 1 R 1 R R R 1 R R 1 R
R R R R 1 R
R 1 R
R 1 R R
R 1 R 1 R R 1
R R 1
R 1 R 1
R 1 R 1 R R
R R 1 R R 1 R 1
R 1 R 1
R R R 1 R R 1 R R 1 R
R R 1 R R R
R 1 R R
R R 1 R R 1 R 1
R 1
R 1
R R 1 R R 1
R 1
R 1 R R
R R R 1 R 1
R 1 R 1
R R 1
R 1 R 1 R R R 1
R R 1 R
R
R 1 R 1 R 1 R 1
R
R 1 R 1 R
R R 1 R 1 R R 1 R 1
R R
R R 1
R 1 R
R 1 R R R 1
R 1
R 1 R 1 R
R 1 R
R R 1 R 1 R 1 R 1
R R R R 1 R 1 R R 1 R 1
R R R R
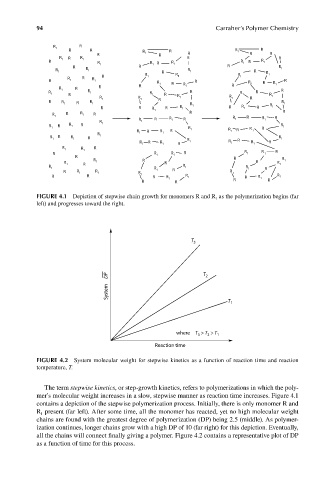
FIGURE 4.1 Depiction of stepwise chain growth for monomers R and R 1 as the polymerization begins (far
left) and progresses toward the right.
T 3
DP T 2
System
T 1
where T > T > T 1
2
3
Reaction time
FIGURE 4.2 System molecular weight for stepwise kinetics as a function of reaction time and reaction
temperature, T.
The term stepwise kinetics, or step-growth kinetics, refers to polymerizations in which the poly-
mer’s molecular weight increases in a slow, stepwise manner as reaction time increases. Figure 4.1
contains a depiction of the stepwise polymerization process. Initially, there is only monomer R and
R present (far left). After some time, all the monomer has reacted, yet no high molecular weight
1
chains are found with the greatest degree of polymerization (DP) being 2.5 (middle). As polymer-
ization continues, longer chains grow with a high DP of 10 (far right) for this depiction. Eventually,
all the chains will connect finally giving a polymer. Figure 4.2 contains a representative plot of DP
as a function of time for this process.
9/14/2010 3:38:03 PM
K10478.indb 94 9/14/2010 3:38:03 PM
K10478.indb 94